Postnatal Nutrient Repartitioning due to Adaptive Developmental Programming
- PMID: 31103181
- PMCID: PMC6527338
- DOI: 10.1016/j.cvfa.2019.02.001
Postnatal Nutrient Repartitioning due to Adaptive Developmental Programming
Abstract
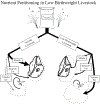
Fetal stress induces developmental adaptations that result in intrauterine growth restriction (IUGR) and low birthweight. These adaptations reappropriate nutrients to the most essential tissues, which benefits fetal survival. The same adaptations are detrimental to growth efficiency and carcass value in livestock, however, because muscle is disproportionally targeted. IUGR adipocytes, liver tissues, and pancreatic β-cells also exhibit functional adaptations. Identifying mechanisms underlying adaptive changes is fundamental to improving outcomes and value in low birthweight livestock. The article outlines studies that have begun to identify stress-induced fetal adaptations affecting growth, metabolism, and differential nutrient utilization in IUGR-born animals.
Keywords: Developmental origins of health and disease; Fetal adaptations; Fetal stress; Nutrient repartitioning; Thrifty phenotype.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures
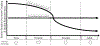
Similar articles
-
ASAS-SSR Triennnial Reproduction Symposium: Looking Back and Moving Forward-How Reproductive Physiology has Evolved: Fetal origins of impaired muscle growth and metabolic dysfunction: Lessons from the heat-stressed pregnant ewe.J Anim Sci. 2018 Jun 29;96(7):2987-3002. doi: 10.1093/jas/sky164. J Anim Sci. 2018. PMID: 29701769 Free PMC article. Review.
-
Board-invited review: intrauterine growth retardation: implications for the animal sciences.J Anim Sci. 2006 Sep;84(9):2316-37. doi: 10.2527/jas.2006-156. J Anim Sci. 2006. PMID: 16908634 Review.
-
Developmental Programming and Growth of Livestock Tissues for Meat Production.Vet Clin North Am Food Anim Pract. 2019 Jul;35(2):303-319. doi: 10.1016/j.cvfa.2019.02.008. Vet Clin North Am Food Anim Pract. 2019. PMID: 31103183 Review.
-
Developmental Programming of Fetal Growth and Development.Vet Clin North Am Food Anim Pract. 2019 Jul;35(2):229-247. doi: 10.1016/j.cvfa.2019.02.006. Vet Clin North Am Food Anim Pract. 2019. PMID: 31103178 Review.
-
Going Up Inflame: Reviewing the Underexplored Role of Inflammatory Programming in Stress-Induced Intrauterine Growth Restricted Livestock.Front Anim Sci. 2021 Nov;2:761421. doi: 10.3389/fanim.2021.761421. Epub 2021 Nov 4. Front Anim Sci. 2021. PMID: 34825243 Free PMC article.
Cited by
-
Genetic regulation and variation of expression of miRNA and mRNA transcripts in fetal muscle tissue in the context of sex, dam and variable fetal weight.Biol Sex Differ. 2022 May 12;13(1):24. doi: 10.1186/s13293-022-00433-3. Biol Sex Differ. 2022. PMID: 35550009 Free PMC article.
-
Sustained maternal inflammation during the early third-trimester yields intrauterine growth restriction, impaired skeletal muscle glucose metabolism, and diminished β-cell function in fetal sheep1,2.J Anim Sci. 2019 Dec 17;97(12):4822-4833. doi: 10.1093/jas/skz321. J Anim Sci. 2019. PMID: 31616931 Free PMC article.
-
Daily Eicosapentaenoic Acid Infusion in IUGR Fetal Lambs Reduced Systemic Inflammation, Increased Muscle ADRβ2 Content, and Improved Myoblast Function and Muscle Growth.Metabolites. 2024 Jun 18;14(6):340. doi: 10.3390/metabo14060340. Metabolites. 2024. PMID: 38921474 Free PMC article.
-
Small for Gestational Age Calves: Part II-Reduced Fertility, Productive Performance, and Survival in Holstein Friesian Heifers Born Small for Their Gestational Age.Animals (Basel). 2024 Jul 24;14(15):2157. doi: 10.3390/ani14152157. Animals (Basel). 2024. PMID: 39123682 Free PMC article.
-
Altered Liver Metabolism, Mitochondrial Function, Oxidative Status, and Inflammatory Response in Intrauterine Growth Restriction Piglets with Different Growth Patterns before Weaning.Metabolites. 2022 Nov 1;12(11):1053. doi: 10.3390/metabo12111053. Metabolites. 2022. PMID: 36355136 Free PMC article.
References
-
- Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35(7):595–601. - PubMed
-
- Funston RN, Summers AF. Effect of prenatal programming on heifer development. Vet Clin North Am Food Anim Pract. 2013;29(3):517–536. - PubMed
-
- Yates DT, Petersen JL, Schmidt TB, Cadaret CN, Barnes TL, Posont RJ, Beede KA. ASAS-SSR Triennnial Reproduction Symposium: Looking Back and Moving Forward- How Reproductive Physiology has Evolved: Fetal origins of impaired muscle growth and metabolic dysfunction: Lessons from the heat-stressed pregnant ewe. J Anim Sci. 2018;96(7):2987–3002. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

