Mobility of Molecular Motors Regulates Contractile Behaviors of Actin Networks
- PMID: 31103238
- PMCID: PMC6554474
- DOI: 10.1016/j.bpj.2019.04.018
Mobility of Molecular Motors Regulates Contractile Behaviors of Actin Networks
Abstract
Cells generate mechanical forces primarily from interactions between F-actin, cross-linking proteins, myosin motors, and other actin-binding proteins in the cytoskeleton. To understand how molecular interactions between the cytoskeletal elements generate forces, a number of in vitro experiments have been performed but are limited in their ability to accurately reproduce the diversity of motor mobility. In myosin motility assays, myosin heads are fixed on a surface and glide F-actin. By contrast, in reconstituted gels, the motion of both myosin and F-actin is unrestricted. Because only these two extreme conditions have been used, the importance of mobility of motors for network behaviors has remained unclear. In this study, to illuminate the impacts of motor mobility on the contractile behaviors of the actin cytoskeleton, we employed an agent-based computational model based on Brownian dynamics. We find that if motors can bind to only one F-actin like myosin I, networks are most contractile at intermediate mobility. In this case, less motor mobility helps motors stably pull F-actins to generate tensile forces, whereas higher motor mobility allows F-actins to aggregate into larger clustering structures. The optimal intermediate motor mobility depends on the stall force and affinity of motors that are regulated by mechanochemical rates. In addition, we find that the role of motor mobility can vary drastically if motors can bind to a pair of F-actins. A network can exhibit large contraction with high motor mobility because motors bound to antiparallel pairs of F-actins can exert similar forces regardless of their mobility. Results from this study imply that the mobility of molecular motors may critically regulate contractile behaviors of actin networks in cells.
Copyright © 2019 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

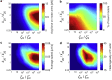

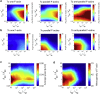


Similar articles
-
Emergence of diverse patterns driven by molecular motors in the motility assay.Cytoskeleton (Hoboken). 2024 Dec;81(12):902-912. doi: 10.1002/cm.21808. Epub 2023 Nov 10. Cytoskeleton (Hoboken). 2024. PMID: 37947256 Free PMC article.
-
Collective and contractile filament motions in the myosin motility assay.Soft Matter. 2020 Feb 12;16(6):1548-1559. doi: 10.1039/c9sm02082a. Soft Matter. 2020. PMID: 31942899 Free PMC article.
-
Dynamic motions of molecular motors in the actin cytoskeleton.Cytoskeleton (Hoboken). 2019 Nov;76(11-12):517-531. doi: 10.1002/cm.21582. Epub 2019 Dec 9. Cytoskeleton (Hoboken). 2019. PMID: 31758841 Free PMC article.
-
Force Generation by Myosin Motors: A Structural Perspective.Chem Rev. 2020 Jan 8;120(1):5-35. doi: 10.1021/acs.chemrev.9b00264. Epub 2019 Nov 5. Chem Rev. 2020. PMID: 31689091 Review.
-
Vesicle transport: the role of actin filaments and myosin motors.Microsc Res Tech. 1999 Oct 15;47(2):93-106. doi: 10.1002/(SICI)1097-0029(19991015)47:2<93::AID-JEMT2>3.0.CO;2-P. Microsc Res Tech. 1999. PMID: 10523788 Review.
Cited by
-
Emergence of a smooth interface from growth of a dendritic network against a mechanosensitive contractile material.Elife. 2021 Aug 23;10:e66929. doi: 10.7554/eLife.66929. Elife. 2021. PMID: 34423780 Free PMC article.
References
-
- Murrell M., Oakes P.W., Gardel M.L. Forcing cells into shape: the mechanics of actomyosin contractility. Nat. Rev. Mol. Cell Biol. 2015;16:486–498. - PMC - PubMed
- Murrell, M., P. W. Oakes, …, M. L. Gardel. 2015. Forcing cells into shape: the mechanics of actomyosin contractility. Nat. Rev. Mol. Cell Biol. 16:486-498. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

