CD55 Is Essential for CD103+ Dendritic Cell Tolerogenic Responses that Protect against Autoimmunity
- PMID: 31103439
- PMCID: PMC6617008
- DOI: 10.1016/j.ajpath.2019.04.008
CD55 Is Essential for CD103+ Dendritic Cell Tolerogenic Responses that Protect against Autoimmunity
Abstract
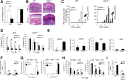
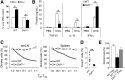
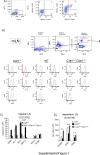
Recent studies traced inflammatory bowel disease in some patients to deficiency of CD55 [decay-accelerating factor (DAF)], but the mechanism underlying the linkage remained unclear. Herein, we studied the importance of DAF in enabling processes that program tolerance in the gut and the eye, two immune-privileged sites where immunosuppressive responses are continuously elicited. Unlike oral feeding or ocular injection of ovalbumin in wild-type (WT) mice, which induced dominant immune tolerance, identical treatment of DAF-/- mice or DAF-/- to WT bone marrow chimeras did not. While 10% to 30% of mesenteric and submandibular lymph node CD4+ cells became robust T-regulatory cells (Tregs) in WT forkhead box P3 (Foxp3)-green fluorescent protein mice, few in either site became Tregs with little suppressor activity in DAF-/- Foxp3-green fluorescent protein mice. Phenotyping of CD103+ dendritic cells (DCs) from the ovalbumin-fed DAF-/- mice showed impaired expression of inducer of costimulation (ICOS) ligand, programmed death receptor 1-ligand 1 (PD1-L1), CxxxC chemokine receptor 1 (Cx3CR1), CCR7, and CCR9. Analyses of elicited DAF-/- Foxp3+ Tregs showed reduced expression of interferon regulatory factor 8 (IRF-8)/aldehyde dehydrogenase 1 family member A2 (Aldh1a2) and glycoprotein A repetitions predominant/latency-associated protein associated with Treg transforming growth factor-β production and presentation, as well as integrin β6/integrin β8 associated with Treg and CD103+ DC transforming growth factor-β release. Thus, DAF is required for the properties of CD103+ DCs and their naïve CD4+ cell partners that together program tolerance.
Copyright © 2019 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Mesenteric lymph node CD11b- CD103+ PD-L1High dendritic cells highly induce regulatory T cells.Immunology. 2017 Sep;152(1):52-64. doi: 10.1111/imm.12747. Epub 2017 Jun 1. Immunology. 2017. PMID: 28423181 Free PMC article.
-
Preferential expression of integrin αvβ8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells.Gastroenterology. 2011 Nov;141(5):1813-20. doi: 10.1053/j.gastro.2011.06.076. Epub 2011 Jul 13. Gastroenterology. 2011. PMID: 21745448 Free PMC article.
-
CD103+ Kidney Dendritic Cells Protect against Crescentic GN by Maintaining IL-10-Producing Regulatory T Cells.J Am Soc Nephrol. 2016 Nov;27(11):3368-3382. doi: 10.1681/ASN.2015080873. Epub 2016 Apr 1. J Am Soc Nephrol. 2016. PMID: 27036736 Free PMC article.
-
Development and functional specialization of CD103+ dendritic cells.Immunol Rev. 2010 Mar;234(1):268-81. doi: 10.1111/j.0105-2896.2009.00874.x. Immunol Rev. 2010. PMID: 20193025 Review.
-
Induction of Immune Tolerance to Dietary Antigens.Adv Exp Med Biol. 2015;850:93-118. doi: 10.1007/978-3-319-15774-0_8. Adv Exp Med Biol. 2015. PMID: 26324349 Review.
Cited by
-
The pathogenic role of retinoid nuclear receptor signaling in cancer and metabolic syndromes.J Exp Med. 2024 Sep 2;221(9):e20240519. doi: 10.1084/jem.20240519. Epub 2024 Aug 12. J Exp Med. 2024. PMID: 39133222 Free PMC article. Review.
-
Vascular Endothelial Cells Produce Coagulation Factors That Control Their Growth via Joint Protease-Activated Receptor and C5a Receptor 1 (CD88) Signaling.Am J Pathol. 2022 Feb;192(2):361-378. doi: 10.1016/j.ajpath.2021.09.011. Am J Pathol. 2022. PMID: 35144762 Free PMC article.
-
Disabled C3ar1/C5ar1 Signaling in Foxp3+ T Regulatory Cells Leads to TSDR Demethylation and Long-Term Stability.J Immunol. 2023 Nov 1;211(9):1359-1366. doi: 10.4049/jimmunol.2300184. J Immunol. 2023. PMID: 37756526 Free PMC article.
-
Gamma-delta T cells modulate the microbiota and fecal micro-RNAs to maintain mucosal tolerance.Microbiome. 2023 Feb 23;11(1):32. doi: 10.1186/s40168-023-01478-1. Microbiome. 2023. PMID: 36814316 Free PMC article.
-
Leveraging U-Net and ASPP for effective fault detection in photovoltaic modules.Sci Rep. 2025 Jul 1;15(1):21788. doi: 10.1038/s41598-025-06646-x. Sci Rep. 2025. PMID: 40596081 Free PMC article.
References
-
- Niederkorn J., Streilein J.W., Shadduck J.A. Deviant immune responses to allogeneic tumors injected intracamerally and subcutaneously in mice. Invest Ophthalmol Vis Sci. 1981;20:355–363. - PubMed
- Niederkorn J, Streilein JW, Shadduck JA: Deviant immune responses to allogeneic tumors injected intracamerally and subcutaneously in mice. Investigative ophthalmology & visual science 1981, 20:355-363. - PubMed
-
- Niederkorn J.Y., Streilein J.W. Alloantigens placed into the anterior chamber of the eye induce specific suppression of delayed-type hypersensitivity but normal cytotoxic T lymphocyte and helper T lymphocyte responses. J Immunol. 1983;131:2670–2674. - PubMed
- Niederkorn JY, Streilein JW: Alloantigens placed into the anterior chamber of the eye induce specific suppression of delayed-type hypersensitivity but normal cytotoxic T lymphocyte and helper T lymphocyte responses. Journal of immunology 1983, 131:2670-2674. - PubMed
-
- McKenna K.C., Xu Y., Kapp J.A. Injection of soluble antigen into the anterior chamber of the eye induces expansion and functional unresponsiveness of antigen-specific CD8+ T cells. J Immunol. 2002;169:5630–5637. - PubMed
- McKenna KC, Xu Y, Kapp JA: Injection of soluble antigen into the anterior chamber of the eye induces expansion and functional unresponsiveness of antigen-specific CD8+ T cells. Journal of immunology 2002, 169:5630-5637. - PubMed
-
- Streilein J.W., Niederkorn J.Y., Shadduck J.A. Systemic immune unresponsiveness induced in adult mice by anterior chamber presentation of minor histocompatibility antigens. J Exp Med. 1980;152:1121–1125. - PMC - PubMed
- Streilein JW, Niederkorn JY, Shadduck JA: Systemic immune unresponsiveness induced in adult mice by anterior chamber presentation of minor histocompatibility antigens. J Exp Med 1980, 152:1121-1125. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

