The Structure of Dental Plaque Microbial Communities in the Transition from Health to Dental Caries and Periodontal Disease
- PMID: 31103772
- PMCID: PMC6646062
- DOI: 10.1016/j.jmb.2019.05.016
The Structure of Dental Plaque Microbial Communities in the Transition from Health to Dental Caries and Periodontal Disease
Abstract
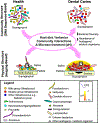
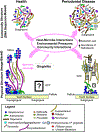
The human oral cavity harbors diverse communities of microbes that live as biofilms: highly ordered, surface-associated assemblages of microbes embedded in an extracellular matrix. Oral microbial communities contribute to human health by fine-tuning immune responses and reducing dietary nitrate. Dental caries and periodontal disease are together the most prevalent microbially mediated human diseases worldwide. Both of these oral diseases are known to be caused not by the introduction of exogenous pathogens to the oral environment, but rather by a homeostasis breakdown that leads to changes in the structure of the microbial communities present in states of health. Both dental caries and periodontal disease are mediated by synergistic interactions within communities, and both diseases are further driven by specific host inputs: diet and behavior in the case of dental caries and immune system interactions in the case of periodontal disease. Changes in community structure (taxonomic identity and abundance) are well documented during the transition from health to disease. In this review, changes in biofilm physical structure during the transition from oral health to disease and the concomitant relationship between structure and community function will be emphasized.
Keywords: dental caries; oral microbiome; periodontal disease; plaque structure; subgingival biofilm.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

