Effect of a Recombinant Human Soluble Thrombomodulin on Mortality in Patients With Sepsis-Associated Coagulopathy: The SCARLET Randomized Clinical Trial
- PMID: 31104069
- PMCID: PMC6547077
- DOI: 10.1001/jama.2019.5358
Effect of a Recombinant Human Soluble Thrombomodulin on Mortality in Patients With Sepsis-Associated Coagulopathy: The SCARLET Randomized Clinical Trial
Abstract
Importance: Previous research suggested that soluble human recombinant thrombomodulin may reduce mortality among patients with sepsis-associated coagulopathy.
Objective: To determine the effect of human recombinant thrombomodulin vs placebo on 28-day all-cause mortality among patients with sepsis-associated coagulopathy.
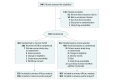
Design, setting, and participants: The SCARLET trial was a randomized, double-blind, placebo-controlled, multinational, multicenter phase 3 study conducted in intensive care units at 159 sites in 26 countries. All adult patients admitted to one of the participating intensive care units between October 2012 and March 2018 with sepsis-associated coagulopathy and concomitant cardiovascular and/or respiratory failure, defined as an international normalized ratio greater than 1.40 without other known etiology and a platelet count in the range of 30 to 150 × 109/L or a greater than 30% decrease in platelet count within 24 hours, were considered for inclusion. The final date of follow-up was February 28, 2019.
Interventions: Patients with sepsis-associated coagulopathy were randomized and treated with an intravenous bolus or a 15-minute infusion of thrombomodulin (0.06 mg/kg/d [maximum, 6 mg/d]; n = 395) or matching placebo (n = 405) once daily for 6 days.
Main outcome and measures: The primary end point was 28-day all-cause mortality.
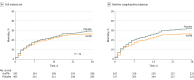
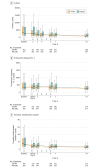
Results: Among 816 randomized patients, 800 (mean age, 60.7 years; 437 [54.6%] men) completed the study and were included in the full analysis set. In these patients, the 28-day all-cause mortality rate was not statistically significantly different between the thrombomodulin group and the placebo group (106 of 395 patients [26.8%] vs 119 of 405 patients [29.4%], respectively; P = .32). The absolute risk difference was 2.55% (95% CI, -3.68% to 8.77%). The incidence of serious major bleeding adverse events (defined as any intracranial hemorrhage; life-threatening bleeding; or bleeding event classified as serious by the investigator, with administration of at least 1440 mL [typically 6 units] of packed red blood cells over 2 consecutive days) was 23 of 396 patients (5.8%) in the thrombomodulin group and 16 of 404 (4.0%) in the placebo group.
Conclusions and relevance: Among patients with sepsis-associated coagulopathy, administration of a human recombinant thrombomodulin, compared with placebo, did not significantly reduce 28-day all-cause mortality.
Trial registration: ClinicalTrials.gov Identifier: NCT01598831.
Conflict of interest statement
Figures
Comment in
-
Recombinant Human Soluble Thrombomodulin in Patients With Sepsis-Associated Coagulopathy: Another Negative Sepsis Trial?JAMA. 2019 May 28;321(20):1978-1980. doi: 10.1001/jama.2019.5792. JAMA. 2019. PMID: 31104068 No abstract available.
-
Recombinant human soluble thrombomodulin in patients with sepsis-associated coagulopathy (SCARLET): an updated meta-analysis.Crit Care. 2019 Sep 5;23(1):302. doi: 10.1186/s13054-019-2587-2. Crit Care. 2019. PMID: 31488189 Free PMC article. No abstract available.
-
Should we continue to test soluble thrombomodulin, or other systemic anticoagulants, as a life-saving therapy for sepsis-induced coagulopathy?Anaesth Crit Care Pain Med. 2019 Oct;38(5):419-421. doi: 10.1016/j.accpm.2019.09.003. Anaesth Crit Care Pain Med. 2019. PMID: 31585759 No abstract available.

