Advanced implantable drug delivery technologies: transforming the clinical landscape of therapeutics for chronic diseases
- PMID: 31104136
- PMCID: PMC7161312
- DOI: 10.1007/s10544-019-0389-6
Advanced implantable drug delivery technologies: transforming the clinical landscape of therapeutics for chronic diseases
Abstract
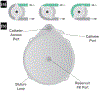
Chronic diseases account for the majority of all deaths worldwide, and their prevalence is expected to escalate in the next 10 years. Because chronic disorders require long-term therapy, the healthcare system must address the needs of an increasing number of patients. The use of new drug administration routes, specifically implantable drug delivery devices, has the potential to reduce treatment-monitoring clinical visits and follow-ups with healthcare providers. Also, implantable drug delivery devices can be designed to maintain drug concentrations in the therapeutic window to achieve controlled, continuous release of therapeutics over extended periods, eliminating the risk of patient non-compliance to oral treatment. A higher local drug concentration can be achieved if the device is implanted in the affected tissue, reducing systemic adverse side effects and decreasing the challenges and discomfort of parenteral treatment. Although implantable drug delivery devices have existed for some time, interest in their therapeutic potential is growing, with a global market expected to reach over $12 billion USD by 2018. This review discusses implantable drug delivery technologies in an advanced stage of development or in clinical use and focuses on the state-of-the-art of reservoir-based implants including pumps, electromechanical systems, and polymers, sites of implantation and side effects, and deployment in developing countries.
Keywords: Implants; Long-acting formulations; MEMS; NEMS; Non-biodegradable polymers.
Conflict of interest statement
Figures








References
-
- Allen EE, Instrument for transfusion of blood with patent 249,285. 8 November 1881.
-
- Allen RH, Kaunitz AM, Hickey M, in Williams Textb. Endocrinol, 13th edn, ed. by Melmed S, Polonsky KS, Larsen PR, Kronenberg HM. (Elsevier, Philadelphia, 2016), pp. 664–693
-
- Almeida DRP, Chin EK, Mears K, Russell SR, Mahajan VB, Retin. Cases Brief Rep 9, 142 (2015) - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

