Fucosyltransferase 4 and 7 mediates adhesion of non-small cell lung cancer cells to brain-derived endothelial cells and results in modification of the blood-brain-barrier: in vitro investigation of CD15 and CD15s in lung-to-brain metastasis
- PMID: 31104223
- PMCID: PMC6591197
- DOI: 10.1007/s11060-019-03188-x
Fucosyltransferase 4 and 7 mediates adhesion of non-small cell lung cancer cells to brain-derived endothelial cells and results in modification of the blood-brain-barrier: in vitro investigation of CD15 and CD15s in lung-to-brain metastasis
Abstract
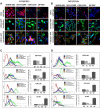
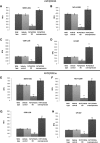
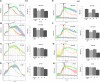
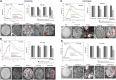
Purpose: Metastatic non-small cell lung (NSCLC) cancer represents one of the most common types of brain metastasis. The mechanisms involved in how circulating cancer cells transmigrate into brain parenchyma are not fully understood. The aim of this work was to investigate the role of fucosylated carbohydrate epitopes CD15 and sialyated CD15s in cancer adhesion to brain-derived endothelial cells and determine their influence in blood-brain barrier (BBB) disruption METHODS: Three distinct, independent methods were used to measure brain endothelial integrity and include voltohmmeter (EVOM™), impedance spectroscopy (CellZscope®) and electric cell-substrate impedance sensing system (ECIS™). Two fucosyltransferases (FUT4 and 7) responsible for CD15 and CD15s synthesis were modulated in four human cancer cell lines (three lung cancer and one glioma).
Results: Overexpression of CD15 or CD15s epitopes led to increase in adhesion of cancer cells to cerebral endothelial cells compared with wild-type and cells with silenced CD15 or CD15s (p < 0.01). This overexpression led to the disruption of cerebral endothelial cell monolayers (p < 0.01). Knockdown of FUT4 and FUT7 in metastatic cancer cells prevented disruption of an in vitro BBB model. Surprisingly, although the cells characterised as 'non-metastatic', they became 'metastatic' -like when cells were forced to over-express either FUT4 or FUT7.
Conclusions: Results from these studies suggest that overexpression of CD15 and CD15s could potentiate the transmigration of circulating NSCLC cells into the brain. The clinical significance of these studies includes the possible use of these epitopes as biomarkers for metastasis.
Keywords: Brain metastasis; CD15; CD15s; FUT4; FUT7; NSCLC.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
CD15s/CD62E Interaction Mediates the Adhesion of Non-Small Cell Lung Cancer Cells on Brain Endothelial Cells: Implications for Cerebral Metastasis.Int J Mol Sci. 2017 Jul 10;18(7):1474. doi: 10.3390/ijms18071474. Int J Mol Sci. 2017. PMID: 28698503 Free PMC article.
-
TNF-α enhancement of CD62E mediates adhesion of non-small cell lung cancer cells to brain endothelium via CD15 in lung-brain metastasis.Neuro Oncol. 2016 May;18(5):679-90. doi: 10.1093/neuonc/nov248. Epub 2015 Oct 15. Neuro Oncol. 2016. PMID: 26472821 Free PMC article.
-
A cloned CD15s-negative variant of HL60 cells is deficient in expression of FUT7 and does not adhere to cytokine-stimulated endothelial cells.Eur J Haematol. 1999 Jul;63(1):42-9. doi: 10.1111/j.1600-0609.1999.tb01849.x. Eur J Haematol. 1999. PMID: 10414454
-
Prognostic and Therapeutic Role of CD15 and CD15s in Cancer.Cancers (Basel). 2022 Apr 28;14(9):2203. doi: 10.3390/cancers14092203. Cancers (Basel). 2022. PMID: 35565333 Free PMC article. Review.
-
Role of the blood-brain barrier in the formation of brain metastases.Int J Mol Sci. 2013 Jan 11;14(1):1383-411. doi: 10.3390/ijms14011383. Int J Mol Sci. 2013. PMID: 23344048 Free PMC article. Review.
Cited by
-
Novel blood-based FUT7 DNA methylation is associated with lung cancer: especially for lung squamous cell carcinoma.Clin Epigenetics. 2022 Dec 3;14(1):167. doi: 10.1186/s13148-022-01389-2. Clin Epigenetics. 2022. PMID: 36463240 Free PMC article.
-
MicroRNAs in Lung Cancer Brain Metastasis.Int J Mol Sci. 2024 Sep 25;25(19):10325. doi: 10.3390/ijms251910325. Int J Mol Sci. 2024. PMID: 39408656 Free PMC article. Review.
-
Abnormal sialylation and fucosylation of saliva glycoproteins: Characteristics of lung cancer-specific biomarkers.Curr Res Pharmacol Drug Discov. 2021 Dec 20;3:100079. doi: 10.1016/j.crphar.2021.100079. eCollection 2022. Curr Res Pharmacol Drug Discov. 2021. PMID: 35005612 Free PMC article. Review.
-
Ceritinib Reduces Transendothelial Invasion of Non-small Cell Lung Cancer Cells by Restoring Claudin-10 and Suppressing VEGF-A Signaling.Biochem Genet. 2025 Apr 21. doi: 10.1007/s10528-025-11103-5. Online ahead of print. Biochem Genet. 2025. PMID: 40259199
-
Exosome-based targeted delivery of NF-κB ameliorates age-related neuroinflammation in the aged mouse brain.Exp Mol Med. 2025 Feb;57(1):235-248. doi: 10.1038/s12276-024-01388-8. Epub 2025 Jan 20. Exp Mol Med. 2025. PMID: 39833561 Free PMC article.
References
-
- Nolan C, De Angelis LM. Overview of metastatic disease of the central nervous system. In: Schiff D, Bent MJ, editors. Handbook of clinical neurology. Amsterdam: Elsevier; 2018. pp. 2–23. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

