Cardiac-specific overexpression of metallothionein attenuates L-NAME-induced myocardial contractile anomalies and apoptosis
- PMID: 31104354
- PMCID: PMC6584723
- DOI: 10.1111/jcmm.14375
Cardiac-specific overexpression of metallothionein attenuates L-NAME-induced myocardial contractile anomalies and apoptosis
Abstract
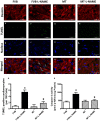
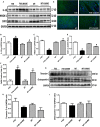
Hypertension contributes to the high cardiac morbidity and mortality. Although oxidative stress plays an essential role in hypertensive heart diseases, the mechanism remains elusive. Transgenic mice with cardiac overexpression of metallothionein, a heavy metal-binding scavenger, were challenged with NG -nitro-L-arginine methyl ester (L-NAME) for 14 days prior to measurement of myocardial contractile and intracellular Ca2+ anomalies as well as cell signalling mechanisms using Western blot and immunofluorescence analysis. L-NAME challenge elicited hypertension, macrophage infiltration, oxidative stress, inflammation and cardiac dysfunction manifested as increased proinflammatory macrophage marker F4/80, interleukin-1β (IL-1β), intracellular production, LV end systolic and diastolic diameters as well as depressed fractional shortening. L-NAME treatment reduced mitochondrial membrane potential (MMP), impaired cardiomyocyte contractile and intracellular Ca2+ properties as evidenced by suppressed peak shortening, maximal velocity of shortening/relengthening, rise in intracellular Ca2+ , along with elevated baseline and peak intracellular Ca2+ . These unfavourable mechanical changes and decreased MMP (except blood pressure and macrophage infiltration) were alleviated by overexpression of metallothionein. Furthermore, the apoptosis markers including BAD, Bax, Caspase 9, Caspase 12 and cleaved Caspase 3 were up-regulated while the anti-apoptotic marker Bcl-2 was decreased by L-NAME treatment. Metallothionein transgene reversed L-NAME-induced changes in Bax, Bcl-2, BAD phosphorylation, Caspase 9, Caspase 12 and cleaved Caspase 3. Our results suggest that metallothionein protects against L-NAME-induced myocardial contractile anomalies in part through inhibition of apoptosis.
Keywords: L-NAME; apoptosis; heart; hypertension; metallothionein.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
The authors declared no conflict of interest for this work.
Figures






Similar articles
-
Cardiac-specific overexpression of metallothionein attenuates myocardial remodeling and contractile dysfunction in l-NAME-induced experimental hypertension: Role of autophagy regulation.Toxicol Lett. 2015 Sep 2;237(2):121-32. doi: 10.1016/j.toxlet.2015.06.005. Epub 2015 Jun 9. Toxicol Lett. 2015. PMID: 26068063
-
Antioxidant metallothionein alleviates endoplasmic reticulum stress-induced myocardial apoptosis and contractile dysfunction.Free Radic Res. 2015 Oct;49(10):1187-98. doi: 10.3109/10715762.2015.1013952. Free Radic Res. 2015. PMID: 25968954
-
Cardiac-specific overexpression of metallothionein rescues against cigarette smoking exposure-induced myocardial contractile and mitochondrial damage.PLoS One. 2013;8(2):e57151. doi: 10.1371/journal.pone.0057151. Epub 2013 Feb 19. PLoS One. 2013. PMID: 23431404 Free PMC article.
-
Cardiac-specific overexpression of metallothionein rescues nicotine-induced cardiac contractile dysfunction and interstitial fibrosis.Toxicol Lett. 2011 Apr 10;202(1):8-14. doi: 10.1016/j.toxlet.2011.01.007. Epub 2011 Jan 14. Toxicol Lett. 2011. PMID: 21238558 Free PMC article.
-
Heavy metal scavenger metallothionein attenuates ER stress-induced myocardial contractile anomalies: role of autophagy.Toxicol Lett. 2014 Mar 21;225(3):333-41. doi: 10.1016/j.toxlet.2013.12.024. Epub 2014 Jan 17. Toxicol Lett. 2014. PMID: 24440343 Free PMC article.
Cited by
-
MiR-490-5p functions as tumor suppressor in childhood neuroblastoma by targeting MYEOV.Hum Cell. 2020 Jan;33(1):261-271. doi: 10.1007/s13577-019-00302-z. Epub 2020 Jan 1. Hum Cell. 2020. PMID: 31894478
-
The Alleviative Effect of Naringin Against Cardiovascular Dysfunction and Remodeling in Hypertensive Rats by Suppressing the Angiotensin II Pathway.Food Sci Nutr. 2025 Jun 19;13(6):e70484. doi: 10.1002/fsn3.70484. eCollection 2025 Jun. Food Sci Nutr. 2025. PMID: 40548179 Free PMC article.
-
Unraveling the Mystery of Cold Stress-Induced Myocardial Injury.Front Physiol. 2020 Nov 5;11:580811. doi: 10.3389/fphys.2020.580811. eCollection 2020. Front Physiol. 2020. PMID: 33250775 Free PMC article. Review.
-
[Effects and cell signaling mechanism of glutamine on rat cardiomyocytes intervened with serum from burned rat].Zhonghua Shao Shang Za Zhi. 2021 Dec 20;37(12):1149-1157. doi: 10.3760/cma.j.cn501120-20210601-00208. Zhonghua Shao Shang Za Zhi. 2021. PMID: 34839600 Free PMC article. Chinese.
-
Beclin1 haploinsufficiency compromises mesenchymal stem cell-offered cardioprotection against myocardial infarction.Cell Regen. 2022 Jun 2;11(1):21. doi: 10.1186/s13619-022-00121-y. Cell Regen. 2022. PMID: 35650374 Free PMC article.
References
-
- Jeemon P, Gupta R, Onen C, et al. Management of Hypertension and Dyslipidemia for Primary Prevention of Cardiovascular Disease In: Prabhakaran D, Anand S, Gaziano TA, Mbanya JC, Wu Y, Nugent R. editors. Washington (DC): Cardiovascular, Respiratory, and Related Disorders, 2017. - PubMed
-
- Hu N, Zhang Y, Nair S, Culver BW, Ren J. Contribution of ALDH2 polymorphism to alcoholism‐associated hypertension. Recent Pat Endocr Metab Immune Drug Discov. 2014;8:180‐185. - PubMed
-
- Wolf M, Ewen S, Mahfoud F, Bohm M. Hypertension: history and development of established and novel treatments. Clin Res Cardiol. 2018;107:16‐29. - PubMed
-
- Li Z, Bing OH, Long X, Robinson KG, Lakatta EG. Increased cardiomyocyte apoptosis during the transition to heart failure in the spontaneously hypertensive rat. Am J Physiol. 1997;272:H2313‐ H2319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

