Results from Phase I Clinical Trial with Intraspinal Injection of Neural Stem Cells in Amyotrophic Lateral Sclerosis: A Long-Term Outcome
- PMID: 31104357
- PMCID: PMC6708070
- DOI: 10.1002/sctm.18-0154
Results from Phase I Clinical Trial with Intraspinal Injection of Neural Stem Cells in Amyotrophic Lateral Sclerosis: A Long-Term Outcome
Abstract
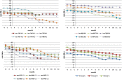
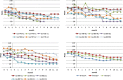
The main objective of this phase I trial was to assess the feasibility and safety of microtransplanting human neural stem cell (hNSC) lines into the spinal cord of patients with amyotrophic lateral sclerosis (ALS). Eighteen patients with a definite diagnosis of ALS received microinjections of hNSCs into the gray matter tracts of the lumbar or cervical spinal cord. Patients were monitored before and after transplantation by clinical, psychological, neuroradiological, and neurophysiological assessment. For up to 60 months after surgery, none of the patients manifested severe adverse effects or increased disease progression because of the treatment. Eleven patients died, and two underwent tracheotomy as a result of the natural history of the disease. We detected a transitory decrease in progression of ALS Functional Rating Scale Revised, starting within the first month after surgery and up to 4 months after transplantation. Our results show that transplantation of hNSC is a safe procedure that causes no major deleterious effects over the short or long term. This study is the first example of medical transplantation of a highly standardized cell drug product, which can be reproducibly and stably expanded ex vivo, comprising hNSC that are not immortalized, and are derived from the forebrain of the same two donors throughout this entire study as well as across future trials. Our experimental design provides benefits in terms of enhancing both intra- and interstudy reproducibility and homogeneity. Given the potential therapeutic effects of the hNSCs, our observations support undertaking future phase II clinical studies in which increased cell dosages are studied in larger cohorts of patients. Stem Cells Translational Medicine 2019;8:887&897.
Keywords: Adult stem cells; Cellular therapy; Clinical trials; Fetal stem cells.
© 2019 The Authors. Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Conflict of interest statement
N.B. is a consultant for NeuralStem and is the patent owner for a device used to transplant the cells (Cleveland Clinic Licensed to NeuralStem). The other authors indicated no potential conflicts of interest.
Figures



References
-
- Brown RH, Al‐Chalabi A. Amyotrophic Lateral Sclerosis. N Engl J Med 2017;377:1602. - PubMed
-
- Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med 1994;330:585–591. - PubMed
-
- Group W, Group EM‐AS . Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: A randomised, double‐blind, placebo‐controlled trial. Lancet Neurol 2017;16:505–512. - PubMed
-
- Vescovi AL, Parati EA, Gritti A et al. Isolation and cloning of multipotential stem cells from the embryonic human CNS and establishment of transplantable human neural stem cell lines by epigenetic stimulation. Exp Neurol 1999;156:71–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

