Length of Uninterrupted CAG, Independent of Polyglutamine Size, Results in Increased Somatic Instability, Hastening Onset of Huntington Disease
- PMID: 31104771
- PMCID: PMC6556907
- DOI: 10.1016/j.ajhg.2019.04.007
Length of Uninterrupted CAG, Independent of Polyglutamine Size, Results in Increased Somatic Instability, Hastening Onset of Huntington Disease
Abstract
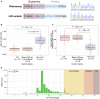
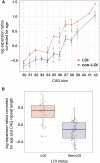
Huntington disease (HD) is caused by a CAG repeat expansion in the huntingtin (HTT) gene. Although the length of this repeat is inversely correlated with age of onset (AOO), it does not fully explain the variability in AOO. We assessed the sequence downstream of the CAG repeat in HTT [reference: (CAG)n-CAA-CAG], since variants within this region have been previously described, but no study of AOO has been performed. These analyses identified a variant that results in complete loss of interrupting (LOI) adenine nucleotides in this region [(CAG)n-CAG-CAG]. Analysis of multiple HD pedigrees showed that this LOI variant is associated with dramatically earlier AOO (average of 25 years) despite the same polyglutamine length as in individuals with the interrupting penultimate CAA codon. This LOI allele is particularly frequent in persons with reduced penetrance alleles who manifest with HD and increases the likelihood of presenting clinically with HD with a CAG of 36-39 repeats. Further, we show that the LOI variant is associated with increased somatic repeat instability, highlighting this as a significant driver of this effect. These findings indicate that the number of uninterrupted CAG repeats, which is lengthened by the LOI, is the most significant contributor to AOO of HD and is more significant than polyglutamine length, which is not altered in these individuals. In addition, we identified another variant in this region, where the CAA-CAG sequence is duplicated, which was associated with later AOO. Identification of these cis-acting modifiers have potentially important implications for genetic counselling in HD-affected families.
Keywords: CAG repeat; Huntington disease; age of onset; genetic modifier; loss of interruption; polyglutamine; reduced penetrance alleles; somatic instability.
Copyright © 2019 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Keum J.W., Shin A., Gillis T., Mysore J.S., Abu Elneel K., Lucente D., Hadzi T., Holmans P., Jones L., Orth M. The HTT CAG-Expansion Mutation Determines Age at Death but Not Disease Duration in Huntington Disease. Am. J. Hum. Genet. 2016;98:287–298. - PMC - PubMed
- Keum, J.W., Shin, A., Gillis, T., Mysore, J.S., Abu Elneel, K., Lucente, D., Hadzi, T., Holmans, P., Jones, L., Orth, M., et al. (2016). The HTT CAG-Expansion Mutation Determines Age at Death but Not Disease Duration in Huntington Disease. Am. J. Hum. Genet. 98, 287-298. - PMC - PubMed
-
- Kay C., Collins J.A., Miedzybrodzka Z., Madore S.J., Gordon E.S., Gerry N., Davidson M., Slama R.A., Hayden M.R. Huntington disease reduced penetrance alleles occur at high frequency in the general population. Neurology. 2016;87:282–288. - PMC - PubMed
- Kay, C., Collins, J.A., Miedzybrodzka, Z., Madore, S.J., Gordon, E.S., Gerry, N., Davidson, M., Slama, R.A., and Hayden, M.R. (2016). Huntington disease reduced penetrance alleles occur at high frequency in the general population. Neurology 87, 282-288. - PMC - PubMed
-
- Maat-Kievit A., Losekoot M., Van Den Boer-Van Den Berg H., Van Ommen G.J., Niermeijer M., Breuning M., Tibben A. New problems in testing for Huntington’s disease: the issue of intermediate and reduced penetrance alleles. J. Med. Genet. 2001;38:E12. - PMC - PubMed
- Maat-Kievit, A., Losekoot, M., Van Den Boer-Van Den Berg, H., Van Ommen, G.J., Niermeijer, M., Breuning, M., and Tibben, A. (2001). New problems in testing for Huntington’s disease: the issue of intermediate and reduced penetrance alleles. J. Med. Genet. 38, E12. - PMC - PubMed
-
- Langbehn D.R., Brinkman R.R., Falush D., Paulsen J.S., Hayden M.R., International Huntington’s Disease Collaborative Group A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin. Genet. 2004;65:267–277. - PubMed
- Langbehn, D.R., Brinkman, R.R., Falush, D., Paulsen, J.S., and Hayden, M.R.; International Huntington’s Disease Collaborative Group (2004). A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin. Genet. 65, 267-277. - PubMed
-
- Wexler N.S., Lorimer J., Porter J., Gomez F., Moskowitz C., Shackell E., Marder K., Penchaszadeh G., Roberts S.A., Gayán J., U.S.-Venezuela Collaborative Research Project Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington’s disease age of onset. Proc. Natl. Acad. Sci. USA. 2004;101:3498–3503. - PMC - PubMed
- Wexler, N.S., Lorimer, J., Porter, J., Gomez, F., Moskowitz, C., Shackell, E., Marder, K., Penchaszadeh, G., Roberts, S.A., Gayan, J., et al.; U.S.-Venezuela Collaborative Research Project (2004). Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington’s disease age of onset. Proc. Natl. Acad. Sci. USA 101, 3498-3503. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

