Human Neonatal Fc Receptor Is the Cellular Uncoating Receptor for Enterovirus B
- PMID: 31104841
- PMCID: PMC7111318
- DOI: 10.1016/j.cell.2019.04.035
Human Neonatal Fc Receptor Is the Cellular Uncoating Receptor for Enterovirus B
Abstract
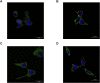
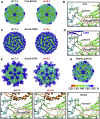
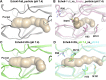
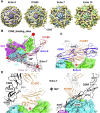
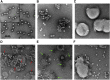
Enterovirus B (EV-B), a major proportion of the genus Enterovirus in the family Picornaviridae, is the causative agent of severe human infectious diseases. Although cellular receptors for coxsackievirus B in EV-B have been identified, receptors mediating virus entry, especially the uncoating process of echovirus and other EV-B remain obscure. Here, we found that human neonatal Fc receptor (FcRn) is the uncoating receptor for major EV-B. FcRn binds to the virus particles in the "canyon" through its FCGRT subunit. By obtaining multiple cryo-electron microscopy structures at different stages of virus entry at atomic or near-atomic resolution, we deciphered the underlying mechanisms of enterovirus attachment and uncoating. These structures revealed that different from the attachment receptor CD55, binding of FcRn to the virions induces efficient release of "pocket factor" under acidic conditions and initiates the conformational changes in viral particle, providing a structural basis for understanding the mechanisms of enterovirus entry.
Keywords: FcRn; attachment; cryo-EM; echovirus; enterovirus; human neonatal Fc receptor; receptor; uncoating.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
W.W. serves as a scientific advisor for EdiGene.
Figures










References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

