Composite Materials and Films Based on Melanins, Polydopamine, and Other Catecholamine-Based Materials
- PMID: 31105175
- PMCID: PMC6352683
- DOI: 10.3390/biomimetics2030012
Composite Materials and Films Based on Melanins, Polydopamine, and Other Catecholamine-Based Materials
Abstract
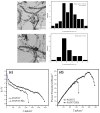
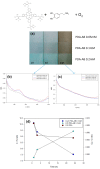
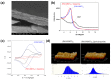
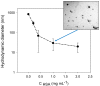
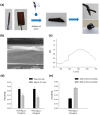
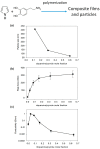
Polydopamine (PDA) is related to eumelanins in its composition and structure. These pigments allow the design, inspired by natural materials, of composite nanoparticles and films for applications in the field of energy conversion and the design of biomaterials. This short review summarizes the main advances in the design of PDA-based composites with inorganic and organic materials.
Keywords: composite films; core-shell nanoparticles; melanin; polydopamine.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Polydopamine and eumelanin: from structure-property relationships to a unified tailoring strategy.Acc Chem Res. 2014 Dec 16;47(12):3541-50. doi: 10.1021/ar500273y. Epub 2014 Oct 23. Acc Chem Res. 2014. PMID: 25340503
-
Melanin-Inspired Composite Materials: From Nanoarchitectonics to Applications.ACS Appl Mater Interfaces. 2024 Jan 24;16(3):3001-3018. doi: 10.1021/acsami.3c14604. Epub 2024 Jan 9. ACS Appl Mater Interfaces. 2024. PMID: 38195388 Review.
-
Polydopamine-Based 3D Colloidal Photonic Materials: Structural Color Balls and Fibers from Melanin-Like Particles with Polydopamine Shell Layers.ACS Appl Mater Interfaces. 2018 Mar 7;10(9):7640-7648. doi: 10.1021/acsami.7b03453. Epub 2017 Jun 29. ACS Appl Mater Interfaces. 2018. PMID: 28661653
-
Biomimetic Chiral Photonic Materials with Tunable Metallic Colorations Prepared from Chiral Melanin-like Nanorods for UV Shielding, Humidity Sensing, and Cosmetics.Langmuir. 2022 Jul 5;38(26):8114-8124. doi: 10.1021/acs.langmuir.2c01004. Epub 2022 Jun 22. Langmuir. 2022. PMID: 35731984
-
Melanin and Melanin-Like Hybrid Materials in Regenerative Medicine.Nanomaterials (Basel). 2020 Aug 3;10(8):1518. doi: 10.3390/nano10081518. Nanomaterials (Basel). 2020. PMID: 32756369 Free PMC article. Review.
Cited by
-
Facile and Controllable Growth of β-FeOOH Nanostructures on Polydopamine Spheres.J Phys Chem B. 2020 Oct 22;124(42):9456-9463. doi: 10.1021/acs.jpcb.0c06627. Epub 2020 Oct 9. J Phys Chem B. 2020. PMID: 32990436 Free PMC article.
-
A Study of the Peculiarities of the Formation of a Hybrid Interface Based on Polydopamine between Dental Tissues and Dental Composites, Using IR and Raman Microspectroscopy, at the Submicron Level.Int J Mol Sci. 2023 Jul 19;24(14):11636. doi: 10.3390/ijms241411636. Int J Mol Sci. 2023. PMID: 37511394 Free PMC article.
-
Bioinspired Catechol-Based Systems: Chemistry and Applications.Biomimetics (Basel). 2017 Dec 20;2(4):25. doi: 10.3390/biomimetics2040025. Biomimetics (Basel). 2017. PMID: 31105186 Free PMC article.
-
Nanostructured Ceria: Biomolecular Templates and (Bio)applications.Nanomaterials (Basel). 2021 Aug 31;11(9):2259. doi: 10.3390/nano11092259. Nanomaterials (Basel). 2021. PMID: 34578575 Free PMC article. Review.
-
Polydopamine Surface Chemistry: A Decade of Discovery.ACS Appl Mater Interfaces. 2018 Mar 7;10(9):7523-7540. doi: 10.1021/acsami.7b19865. Epub 2018 Feb 26. ACS Appl Mater Interfaces. 2018. PMID: 29465221 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

