2- S-Lipoylcaffeic Acid, a Natural Product-Based Entry to Tyrosinase Inhibition via Catechol Manipulation
- PMID: 31105178
- PMCID: PMC6352668
- DOI: 10.3390/biomimetics2030015
2- S-Lipoylcaffeic Acid, a Natural Product-Based Entry to Tyrosinase Inhibition via Catechol Manipulation
Abstract
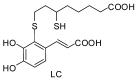
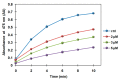
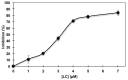
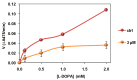
Conjugation of naturally occurring catecholic compounds with thiols is a versatile and facile entry to a broad range of bioinspired multifunctional compounds for diverse applications in biomedicine and materials science. We report herein the inhibition properties of the caffeic acid- dihydrolipoic acid S-conjugate, 2-S-lipoylcaffeic acid (LC), on mushroom tyrosinase. Half maximum inhibitory concentration (IC50) values of 3.22 ± 0.02 and 2.0 ± 0.1 µM were determined for the catecholase and cresolase activity of the enzyme, respectively, indicating a greater efficiency of LC compared to the parent caffeic acid and the standard inhibitor kojic acid. Analysis of the Lineweaver⁻Burk plot suggested a mixed-type inhibition mechanism. LC proved to be non-toxic on human keratinocytes (HaCaT) at concentrations up to 30 µM. These results would point to LC as a novel prototype of melanogenesis regulators for the treatment of pigmentary disorders.
Keywords: ">l-DOPA; caffeic acid; depigmenting agents; dihydrolipoic acid; dopachrome; keratinocytes; lipoic acid; melanin; tyrosinase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources

