Kaxiras's Porphyrin: DFT Modeling of Redox-Tuned Optical and Electronic Properties in a Theoretically Designed Catechol-Based Bioinspired Platform
- PMID: 31105182
- PMCID: PMC6352670
- DOI: 10.3390/biomimetics2040021
Kaxiras's Porphyrin: DFT Modeling of Redox-Tuned Optical and Electronic Properties in a Theoretically Designed Catechol-Based Bioinspired Platform
Abstract
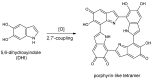
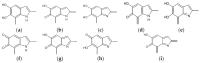
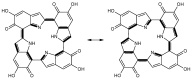
A detailed computational investigation of the 5,6-dihydroxyindole (DHI)-based porphyrin-type tetramer first described by Kaxiras as a theoretical structural model for eumelanin biopolymers is reported herein, with a view to predicting the technological potential of this unique bioinspired tetracatechol system. All possible tautomers/conformers, as well as alternative protonation states, were explored for the species at various degrees of oxidation and all structures were geometry optimized at the density functional theory (DFT) level. Comparison of energy levels for each oxidized species indicated a marked instability of most oxidation states except the six-electron level, and an unexpected resilience to disproportionation of the one-electron oxidation free radical species. Changes in the highest energy occupied molecular orbital (HOMO)⁻lowest energy unoccupied molecular orbital (LUMO) gaps with oxidation state and tautomerism were determined along with the main electronic transitions: more or less intense absorption in the visible region is predicted for most oxidized species. Data indicated that the peculiar symmetry of the oxygenation pattern pertaining to the four catechol/quinone/quinone methide moieties, in concert with the NH centers, fine-tunes the optical and electronic properties of the porphyrin system. For several oxidation levels, conjugated systems extending over two or more indole units play a major role in determining the preferred tautomeric state: thus, the highest stability of the six-electron oxidation state reflects porphyrin-type aromaticity. These results provide new clues for the design of innovative bioinspired optoelectronic materials.
Keywords: DFT; ab initio calculation; eumelanin; porphyrin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
DFT/TD-semiempirical study on the structural and electronic properties and absorption spectra of supramolecular fullerene-porphyrine-metalloporphyrine triads based dye-sensitized solar cells.Spectrochim Acta A Mol Biomol Spectrosc. 2018 Apr 5;194:57-66. doi: 10.1016/j.saa.2017.12.073. Epub 2018 Jan 2. Spectrochim Acta A Mol Biomol Spectrosc. 2018. PMID: 29324256
-
Stability, Aromaticity, and Photophysical Behaviors of Macrocyclic Molecules: A Theoretical Analysis.Front Chem. 2020 Sep 4;8:776. doi: 10.3389/fchem.2020.00776. eCollection 2020. Front Chem. 2020. PMID: 33102432 Free PMC article.
-
Lack of visible chromophore development in the pulse radiolysis oxidation of 5,6-dihydroxyindole-2-carboxylic acid oligomers: DFT investigation and implications for eumelanin absorption properties.J Org Chem. 2009 May 15;74(10):3727-34. doi: 10.1021/jo900250v. J Org Chem. 2009. PMID: 19385623
-
Structural Basis of Polydopamine Film Formation: Probing 5,6-Dihydroxyindole-Based Eumelanin Type Units and the Porphyrin Issue.ACS Appl Mater Interfaces. 2018 Mar 7;10(9):7670-7680. doi: 10.1021/acsami.7b09662. Epub 2017 Sep 29. ACS Appl Mater Interfaces. 2018. PMID: 28937213
-
Structural effects on the electronic absorption properties of 5,6-dihydroxyindole oligomers: the potential of an integrated experimental and DFT approach to model eumelanin optical properties.Photochem Photobiol. 2008 May-Jun;84(3):600-7. doi: 10.1111/j.1751-1097.2007.00249.x. Photochem Photobiol. 2008. PMID: 18435616
Cited by
-
Bioinspired Catechol-Based Systems: Chemistry and Applications.Biomimetics (Basel). 2017 Dec 20;2(4):25. doi: 10.3390/biomimetics2040025. Biomimetics (Basel). 2017. PMID: 31105186 Free PMC article.
-
The Late Stages of Melanogenesis: Exploring the Chemical Facets and the Application Opportunities.Int J Mol Sci. 2018 Jun 13;19(6):1753. doi: 10.3390/ijms19061753. Int J Mol Sci. 2018. PMID: 29899264 Free PMC article. Review.
References
-
- d’Ischia M., Wakamatsu K., Cicoira F., Di Mauro E., Garcia-Borron J.C., Commo S., Galva’n I., Ghanem G., Kenzo K., Meredith P., et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigm. Cell Melanoma Res. 2015;28:520–544. doi: 10.1111/pcmr.12393. - DOI - PubMed
LinkOut - more resources
Full Text Sources

