Synthesis and Characterization of Acetic Acid-Doped Polyaniline and Polyaniline⁻Chitosan Composite
- PMID: 31105200
- PMCID: PMC6477596
- DOI: 10.3390/biomimetics4010015
Synthesis and Characterization of Acetic Acid-Doped Polyaniline and Polyaniline⁻Chitosan Composite
Abstract
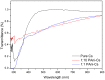
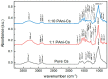
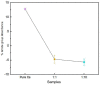
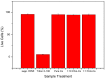
Polyaniline-chitosan (PAni-Cs) composite films were synthesized using a solution casting method with varying PAni concentrations. Polyaniline powders used in the composite synthesis were polymerized using acetic acid as the dopant media. Raman spectroscopy revealed that the PAni powders synthesized using hydrochloric acid and acetic acid did not exhibit significant difference to the chemical features of PAni, implying that PAni was formed in varying concentrations of the dopant media. The presence of agglomerated particles on the surface of the Cs composite, which may have been due to the presence of PAni powders, was observed with scanning electron microscope-energy dispersive X-ray spectroscopy (SEM-EDX). Ultraviolet-visible (UV-Vis) spectroscopy further showed the interaction of PAni with Cs where the Cs characteristic peak shifted to a higher wavelength. Cell viability assay also revealed that the synthesized PAni-Cs composites were nontoxic and may be utilized for future biomedical applications.
Keywords: chitosan; composite; emeraldine; polyaniline; trypan blue assay.
Conflict of interest statement
The authors declare no conflict of interest in this study.
Figures





References
-
- Taaca K.L.M., Vasquez M.R., Jr. Hemocompatibility and cytocompatibility of pristine and plasma-treated silver-zeolite-chitosan composites. Appl. Surf. Sci. 2018;432:324–331. doi: 10.1016/j.apsusc.2017.04.034. - DOI
-
- He W., Benson R. Handbook of Polymer Applications in Medicine and Medical Devices. 1st ed. William Andrew; Oxford, UK: 2013. pp. 55–76.
-
- Pornanong A. Introduction to biomaterials for wound healing. Wound Heal. Biomater. 2016;2:3–38. doi: 10.1016/B978-1-78242-456-7.00001-5. - DOI
-
- O’Brien F.J. Biomaterials and scaffolds for tissue engineering. Mater. Today. 2011;14:88–95. doi: 10.1016/S1369-7021(11)70058-X. - DOI
-
- Taaca K.L.M., Vasquez M.R., Jr. Fabrication of Ag-exchanged zeolite/chitosan composites and effects of plasma treatment. Microporous Mesoporous Mater. 2017;241:383–391. doi: 10.1016/j.micromeso.2017.01.002. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

