Neuromechanical Model of Rat Hindlimb Walking with Two-Layer CPGs
- PMID: 31105206
- PMCID: PMC6477610
- DOI: 10.3390/biomimetics4010021
Neuromechanical Model of Rat Hindlimb Walking with Two-Layer CPGs
Abstract
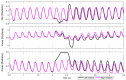
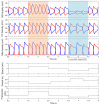
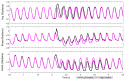
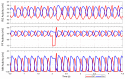
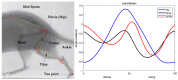
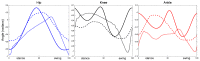
This work demonstrates a neuromechanical model of rat hindlimb locomotion undergoing nominal walking with perturbations. In the animal, two types of responses to perturbations are observed: resetting and non-resetting deletions. This suggests that the animal locomotor system contains a memory-like organization. To model this phenomenon, we built a synthetic nervous system that uses separate rhythm generator and pattern formation layers to activate antagonistic muscle pairs about each joint in the sagittal plane. Our model replicates the resetting and non-resetting deletions observed in the animal. In addition, in the intact (i.e., fully afferented) rat walking simulation, we observe slower recovery after perturbation, which is different from the deafferented animal experiment. These results demonstrate that our model is a biologically feasible description of some of the neural circuits in the mammalian spinal cord that control locomotion, and the difference between our simulation and fictive motion shows the importance of sensory feedback on motor output. This model also demonstrates how the pattern formation network can activate muscle synergies in a coordinated way to produce stable walking, which motivates the use of more complex synergies activating more muscles in the legs for three-dimensional limb motion.
Keywords: muscle synergies; pattern formation; rat; rhythm generator; synthetic nervous system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Evaluating functional roles of phase resetting in generation of adaptive human bipedal walking with a physiologically based model of the spinal pattern generator.Biol Cybern. 2010 May;102(5):373-87. doi: 10.1007/s00422-010-0373-y. Epub 2010 Mar 9. Biol Cybern. 2010. PMID: 20217427
-
Contribution of Phase Resetting to Adaptive Rhythm Control in Human Walking Based on the Phase Response Curves of a Neuromusculoskeletal Model.Front Neurosci. 2020 Feb 5;14:17. doi: 10.3389/fnins.2020.00017. eCollection 2020. Front Neurosci. 2020. PMID: 32116492 Free PMC article.
-
EFFECTS OF SPINAL TRANSECTION AND LOCOMOTOR SPEED ON MUSCLE SYNERGIES OF THE CAT HINDLIMB.bioRxiv [Preprint]. 2024 Sep 20:2024.09.19.613891. doi: 10.1101/2024.09.19.613891. bioRxiv. 2024. Update in: J Physiol. 2025 May;603(10):3061-3088. doi: 10.1113/JP288089. PMID: 39345603 Free PMC article. Updated. Preprint.
-
The Human Central Pattern Generator for Locomotion: Does It Exist and Contribute to Walking?Neuroscientist. 2017 Dec;23(6):649-663. doi: 10.1177/1073858417699790. Epub 2017 Mar 28. Neuroscientist. 2017. PMID: 28351197 Review.
-
Chapter 2--the spinal generation of phases and cycle duration.Prog Brain Res. 2011;188:15-29. doi: 10.1016/B978-0-444-53825-3.00007-3. Prog Brain Res. 2011. PMID: 21333800 Review.
Cited by
-
A three-dimensional musculoskeletal model of the dog.Sci Rep. 2021 May 31;11(1):11335. doi: 10.1038/s41598-021-90058-0. Sci Rep. 2021. PMID: 34059703 Free PMC article.
-
Biomechanical and Sensory Feedback Regularize the Behavior of Different Locomotor Central Pattern Generators.Biomimetics (Basel). 2022 Dec 4;7(4):226. doi: 10.3390/biomimetics7040226. Biomimetics (Basel). 2022. PMID: 36546926 Free PMC article.
-
Stable Gastric Pentadecapeptide BPC 157 as a Therapy for the Disable Myotendinous Junctions in Rats.Biomedicines. 2021 Oct 27;9(11):1547. doi: 10.3390/biomedicines9111547. Biomedicines. 2021. PMID: 34829776 Free PMC article.
-
Incorporating buccal mass planar mechanics and anatomical features improves neuromechanical modeling of Aplysia feeding behavior.Biol Cybern. 2025 Jul 7;119(4-6):17. doi: 10.1007/s00422-025-01017-1. Biol Cybern. 2025. PMID: 40622423 Free PMC article.
References
-
- Brown T.G. On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. J. Physiol. 1914;48:18–46. doi: 10.1113/jphysiol.1914.sp001646. - DOI - PMC - PubMed
-
- Wenger N., Moraud E.M., Gandar J., Musienko P., Capogrosso M., Baud L., Le Goff C.G., Barraud Q., Pavlova N., Dominici N., et al. Spatiotemporal neuromodulation therapies engaging muscle synergies improve motor control after spinal cord injury. Nat. Med. 2016;22:138–145. doi: 10.1038/nm.4025. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

