Synthesis and Mechanochemical Activity of Peptide-Based Cu(I) Bis(N-heterocyclic carbene) Complexes
- PMID: 31105209
- PMCID: PMC6477612
- DOI: 10.3390/biomimetics4010024
Synthesis and Mechanochemical Activity of Peptide-Based Cu(I) Bis(N-heterocyclic carbene) Complexes
Abstract
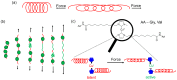
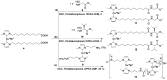
With the class of shock-absorbing proteins, nature created some of the most robust materials combining both mechanical strength and elasticity. Their excellent ability to dissipate energy to prevent surrounding cells from damage is an interesting property that regularly is exploited for applications in biomimetic materials. Similar to biomaterials, where mechanical stimuli are transmitted into a (bio)chemical response, mechanophoric catalysts transform mechanical energy into a chemical reaction. Force transmission is realized commonly by polymeric handles directing the applied force to the mechanophoric bond, which in turn leads to stress-induced activation of the catalyst. Therefore, shock-absorbing proteins able to take up and store mechanical energy elastically for subsequent force transduction to the labile bond seem to be perfect candidates to fulfill this task. Here, we report on the synthesis of two different latent mechanophoric copper(I) bis(N-heterocyclic carbene) complexes bearing either two carboxyl groups or two amino groups which allow conjugation reactions with either the N- or the C-terminus of amino acids or peptides. The chosen catalysts can be activated, for instance, by applying external mechanical force via ultrasound, removing one N-heterocyclic carbene (NHC) ligand. Post-modification of the mechanophoric catalysts via peptide coupling (Gly, Val) and first reactions showed that the mechanoresponsive behavior was still present after the coupling. Subsequent polycondensation of both catalysts lead to a polyamide including the Cu(I) moiety. Mechanochemical activation by ultrasound showed conversions in the copper(I)-catalyzed alkyne-azide "click" reaction (CuAAC) up to 9.9% proving the potential application for the time and spatial controlled CuAAC.
Keywords: copper(I); mechanocatalysts; mechanochemistry; peptide coupling; “click” chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
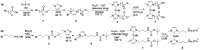




Similar articles
-
Mechanochemical Activation of Fluorogenic CuAAC "Click" Reactions for Stress-Sensing Applications.Macromol Rapid Commun. 2018 Nov;39(22):e1800376. doi: 10.1002/marc.201800376. Epub 2018 Aug 12. Macromol Rapid Commun. 2018. PMID: 30101432
-
A Mechanochemically Triggered "Click" Catalyst.Angew Chem Int Ed Engl. 2015 Nov 16;54(47):13918-22. doi: 10.1002/anie.201505678. Epub 2015 Sep 30. Angew Chem Int Ed Engl. 2015. PMID: 26420664
-
C-X (X = N, O) Cross-Coupling Reactions Catalyzed by Copper-Pincer Bis(N-Heterocyclic Carbene) Complexes.Front Chem. 2019 Jan 31;7:12. doi: 10.3389/fchem.2019.00012. eCollection 2019. Front Chem. 2019. PMID: 30766865 Free PMC article.
-
Ralph F. Hirschmann award address 2009: Merger of organic chemistry with peptide diversity.Biopolymers. 2010;94(2):161-82. doi: 10.1002/bip.21344. Biopolymers. 2010. PMID: 20225304 Review.
-
Mechanosensing and mechanochemical transduction: how is mechanical energy sensed and converted into chemical energy in an extracellular matrix?Crit Rev Biomed Eng. 2003;31(4):255-331. doi: 10.1615/critrevbiomedeng.v31.i4.10. Crit Rev Biomed Eng. 2003. PMID: 15095950 Review.
Cited by
-
Activation of a Copper Biscarbene Mechano-Catalyst Using Single-Molecule Force Spectroscopy Supported by Quantum Chemical Calculations.Chemistry. 2021 Jun 16;27(34):8723-8729. doi: 10.1002/chem.202100555. Epub 2021 May 11. Chemistry. 2021. PMID: 33822419 Free PMC article.
References
-
- Staudinger H., Bondy H.F. Über Isopren und Kautschuk, 19. Mitteil.: Über die Molekülgröße des Kautschuks und der Balata. Chem. Ber. 1930;63:734–736. doi: 10.1002/cber.19300630330. - DOI
-
- Kauzmann W., Eyring H. The viscous flow of large molecules. J. Am. Chem. Soc. 1940;62:3113–3125. doi: 10.1021/ja01868a059. - DOI
-
- Groote R., Jakobs R.T.M., Sijbesma R.P. Mechanocatalysis: Forcing latent catalysts into action. Polym. Chem. 2013;4:4846–4859. doi: 10.1039/c3py00071k. - DOI
-
- Boulatov R. Polymer Mechanochemistry. Volume 369 Springer International Publishing; Heidelberg, Germany: 2015.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

