Hydrogel Actuators and Sensors for Biomedical Soft Robots: Brief Overview with Impending Challenges
- PMID: 31105237
- PMCID: PMC6352708
- DOI: 10.3390/biomimetics3030015
Hydrogel Actuators and Sensors for Biomedical Soft Robots: Brief Overview with Impending Challenges
Abstract
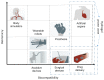
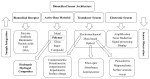
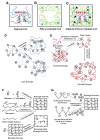
There are numerous developments taking place in the field of biorobotics, and one such recent breakthrough is the implementation of soft robots-a pathway to mimic nature's organic parts for research purposes and in minimally invasive surgeries as a result of their shape-morphing and adaptable features. Hydrogels (biocompatible, biodegradable materials that are used in designing soft robots and sensor integration), have come into demand because of their beneficial properties, such as high water content, flexibility, and multi-faceted advantages particularly in targeted drug delivery, surgery and biorobotics. We illustrate in this review article the different types of biomedical sensors and actuators for which a hydrogel acts as an active primary material, and we elucidate their limitations and the future scope of this material in the nexus of similar biomedical avenues.
Keywords: biomedical sensors; hydrogel robots; soft actuators; stimuli-responsive hydrogels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
Programmable Morphing Hydrogels for Soft Actuators and Robots: From Structure Designs to Active Functions.Acc Chem Res. 2022 Jun 7;55(11):1533-1545. doi: 10.1021/acs.accounts.2c00046. Epub 2022 Apr 12. Acc Chem Res. 2022. PMID: 35413187
-
Nanocomposite Hydrogel Actuators with Ordered Structures: From Nanoscale Control to Macroscale Deformations.Small Methods. 2024 Apr;8(4):e2300414. doi: 10.1002/smtd.202300414. Epub 2023 Jun 27. Small Methods. 2024. PMID: 37365950 Review.
-
Advances in biomimetic stimuli responsive soft grippers.Nano Converg. 2019 Jul 1;6(1):20. doi: 10.1186/s40580-019-0191-4. Nano Converg. 2019. PMID: 31257552 Free PMC article. Review.
-
Muscle-like hydrogels with fast isochoric responses and their applications as soft robots: a minireview.Mater Horiz. 2025 Feb 3;12(3):719-733. doi: 10.1039/d4mh01187b. Mater Horiz. 2025. PMID: 39530734 Review.
-
Hydrogel-Based Continuum Soft Robots.Gels. 2025 Mar 27;11(4):254. doi: 10.3390/gels11040254. Gels. 2025. PMID: 40277689 Free PMC article. Review.
Cited by
-
Surface Deformation of Biocompatible Materials: Recent Advances in Biological Applications.Biomimetics (Basel). 2024 Jun 28;9(7):395. doi: 10.3390/biomimetics9070395. Biomimetics (Basel). 2024. PMID: 39056836 Free PMC article. Review.
-
Specialty Tough Hydrogels and Their Biomedical Applications.Adv Healthc Mater. 2020 Jan;9(2):e1901396. doi: 10.1002/adhm.201901396. Epub 2019 Dec 17. Adv Healthc Mater. 2020. PMID: 31846228 Free PMC article. Review.
-
Two-Photon Polymerization of Albumin Hydrogel Nanowires Strengthened with Graphene Oxide.Biomimetics (Basel). 2021 Nov 24;6(4):66. doi: 10.3390/biomimetics6040066. Biomimetics (Basel). 2021. PMID: 34842608 Free PMC article.
-
Multi-domain automated patterning of DNA-functionalized hydrogels.PLoS One. 2024 Feb 2;19(2):e0295923. doi: 10.1371/journal.pone.0295923. eCollection 2024. PLoS One. 2024. PMID: 38306330 Free PMC article.
-
Mechanically Diverse Gels with Equal Solvent Content.ACS Cent Sci. 2022 Jun 22;8(6):845-852. doi: 10.1021/acscentsci.2c00472. Epub 2022 Jun 9. ACS Cent Sci. 2022. PMID: 35756385 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

