The Chemistry of Polydopamine Film Formation: The Amine-Quinone Interplay
- PMID: 31105248
- PMCID: PMC6352855
- DOI: 10.3390/biomimetics3030026
The Chemistry of Polydopamine Film Formation: The Amine-Quinone Interplay
Abstract
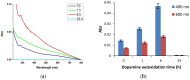
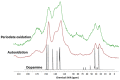
Despite extensive investigations over the past decade, the chemical basis of the extraordinary underwater adhesion properties of polydopamine (PDA) has remained not entirely understood. The bulk of evidence points to PDA wet adhesion as a complex process based on film deposition, and growth in which primary amine groups, besides catechol moieties, play a central role. However, the detailed interplay of chemical interactions underlying the dynamics of film formation has not yet been elucidated. Herein, we report the results of a series of experiments showing that coating formation from dopamine at pH 9.0 in carbonate buffer: (a) Requires high dopamine concentrations (>1 mM); (b) is due to species produced in the early stages of dopamine autoxidation; (c) is accelerated by equimolar amounts of periodate causing fast conversion to the o-quinone; and (d) is enhanced by the addition of hexamethylenediamine (HMDA) and other long chain aliphatic amines even at low dopamine concentrations (<1 mM). It is proposed that concentration-dependent PDA film formation reflects the competition between intermolecular amine-quinone condensation processes, leading to adhesive cross-linked oligomer structures, and the intramolecular cyclization route forming little adhesive 5,6-dihydroxyindole (DHI) units. Film growth would then be sustained by dopamine and other soluble species that can be adsorbed on the surface.
Keywords: amines; film; periodate; polydopamine; polymerization; quinone.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures







Similar articles
-
Hexamethylenediamine-Mediated Polydopamine Film Deposition: Inhibition by Resorcinol as a Strategy for Mapping Quinone Targeting Mechanisms.Front Chem. 2019 Jun 5;7:407. doi: 10.3389/fchem.2019.00407. eCollection 2019. Front Chem. 2019. PMID: 31231635 Free PMC article.
-
Structural Basis of Polydopamine Film Formation: Probing 5,6-Dihydroxyindole-Based Eumelanin Type Units and the Porphyrin Issue.ACS Appl Mater Interfaces. 2018 Mar 7;10(9):7670-7680. doi: 10.1021/acsami.7b09662. Epub 2017 Sep 29. ACS Appl Mater Interfaces. 2018. PMID: 28937213
-
Direct Evidence for the Critical Role of 5,6-Dihydroxyindole in Polydopamine Deposition and Aggregation.Langmuir. 2019 Apr 16;35(15):5191-5201. doi: 10.1021/acs.langmuir.9b00392. Epub 2019 Apr 4. Langmuir. 2019. PMID: 30916980
-
In situ insights into the nanoscale deposition of 5,6-dihydroxyindole-based coatings and the implications on the underwater adhesion mechanism of polydopamine coatings.RSC Adv. 2018 Aug 3;8(49):27695-27702. doi: 10.1039/c8ra04472d. eCollection 2018 Aug 2. RSC Adv. 2018. PMID: 35542737 Free PMC article.
-
Non-covalent small molecule partnership for redox-active films: Beyond polydopamine technology.J Colloid Interface Sci. 2022 Oct 15;624:400-410. doi: 10.1016/j.jcis.2022.05.123. Epub 2022 May 22. J Colloid Interface Sci. 2022. PMID: 35671617
Cited by
-
Reaction-Based, Fluorescent Film Deposition from Dopamine and a Diamine-Tethered, Bis-Resorcinol Coupler.Int J Mol Sci. 2019 Sep 13;20(18):4532. doi: 10.3390/ijms20184532. Int J Mol Sci. 2019. PMID: 31540228 Free PMC article.
-
Bio-Inspired Redox-Adhesive Polydopamine Matrix for Intact Bacteria Biohybrid Photoanodes.ACS Appl Mater Interfaces. 2022 Jun 15;14(23):26631-26641. doi: 10.1021/acsami.2c02410. Epub 2022 May 31. ACS Appl Mater Interfaces. 2022. PMID: 35639658 Free PMC article.
-
Polydopamine Ultrathin Film Growth on Mica via In-Situ Polymerization of Dopamine with Applications for Silver-Based Antimicrobial Coatings.Materials (Basel). 2021 Feb 1;14(3):671. doi: 10.3390/ma14030671. Materials (Basel). 2021. PMID: 33535625 Free PMC article.
-
Bioinspired Nanoplatforms: Polydopamine and Exosomes for Targeted Antimicrobial Therapy.Polymers (Basel). 2025 Jun 16;17(12):1670. doi: 10.3390/polym17121670. Polymers (Basel). 2025. PMID: 40574198 Free PMC article. Review.
-
Recent Advances in a Polydopamine-Mediated Antimicrobial Adhesion System.Front Microbiol. 2021 Jan 12;11:607099. doi: 10.3389/fmicb.2020.607099. eCollection 2020. Front Microbiol. 2021. PMID: 33510726 Free PMC article. Review.
References
-
- Hong S., Na Y.S., Choi S., Song I.T., Kim W.Y., Lee H. Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv. Funct. Mater. 2012;22:4711–4717. doi: 10.1002/adfm.201201156. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

