Straining Flow Spinning of Artificial Silk Fibers: A Review
- PMID: 31105251
- PMCID: PMC6352662
- DOI: 10.3390/biomimetics3040029
Straining Flow Spinning of Artificial Silk Fibers: A Review
Abstract
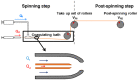
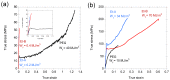
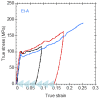
This work summarizes the main principles and some of the most significant results of straining flow spinning (SFS), a technology developed originally by the authors of this work. The principles on which the technology is based, inspired by the natural spinning system of silkworms and spiders, are presented, as well as some of the main achievements of the technique. Among these achievements, spinning under environmentally friendly conditions, obtaining high-performance fibers, and imparting the fibers with emerging properties such as supercontraction are discussed. Consequently, SFS appears as an efficient process that may represent one of the first realizations of a biomimetic technology with a significant impact at the production level.
Keywords: fibroin; regenerated fibers; silk; spidroin.
Conflict of interest statement
The following authors appear as inventors of the patent (see Section 8): J.P.-R., R.M., A.M.G.-C., G.R.P., G.V.G. and M.E.
Figures





References
-
- Kaplan D.L., Lombardi S., Muller W.S., Fossey S.A. Biomaterials. Novel Materials from Biological Sources. Stockton Press; New York, NY, USA: 1991.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

