Gi/o-Protein Coupled Receptors in the Aging Brain
- PMID: 31105551
- PMCID: PMC6492497
- DOI: 10.3389/fnagi.2019.00089
Gi/o-Protein Coupled Receptors in the Aging Brain
Abstract
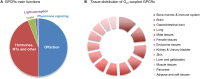
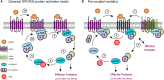
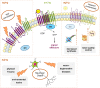
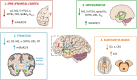
Cells translate extracellular signals to regulate processes such as differentiation, metabolism and proliferation, via transmembranar receptors. G protein-coupled receptors (GPCRs) belong to the largest family of transmembrane receptors, with over 800 members in the human species. Given the variety of key physiological functions regulated by GPCRs, these are main targets of existing drugs. During normal aging, alterations in the expression and activity of GPCRs have been observed. The central nervous system (CNS) is particularly affected by these alterations, which results in decreased brain functions, impaired neuroregeneration, and increased vulnerability to neuropathologies, such as Alzheimer's and Parkinson diseases. GPCRs signal via heterotrimeric G proteins, such as Go, the most abundant heterotrimeric G protein in CNS. We here review age-induced effects of GPCR signaling via the Gi/o subfamily at the CNS. During the aging process, a reduction in protein density is observed for almost half of the Gi/o-coupled GPCRs, particularly in age-vulnerable regions such as the frontal cortex, hippocampus, substantia nigra and striatum. Gi/o levels also tend to decrease with aging, particularly in regions such as the frontal cortex. Alterations in the expression and activity of GPCRs and coupled G proteins result from altered proteostasis, peroxidation of membranar lipids and age-associated neuronal degeneration and death, and have impact on aging hallmarks and age-related neuropathologies. Further, due to oligomerization of GPCRs at the membrane and their cooperative signaling, down-regulation of a specific Gi/o-coupled GPCR may affect signaling and drug targeting of other types/subtypes of GPCRs with which it dimerizes. Gi/o-coupled GPCRs receptorsomes are thus the focus of more effective therapeutic drugs aiming to prevent or revert the decline in brain functions and increased risk of neuropathologies at advanced ages.
Keywords: G protein-coupled receptors GPCRs; Gi/o heterotrimeric G proteins; aging; basal ganglia; frontal cortex; hippocampus; receptor density and binding potential.
Figures
Similar articles
-
Gαi/o-coupled receptor signaling restricts pancreatic β-cell expansion.Proc Natl Acad Sci U S A. 2015 Mar 3;112(9):2888-93. doi: 10.1073/pnas.1319378112. Epub 2015 Feb 18. Proc Natl Acad Sci U S A. 2015. PMID: 25695968 Free PMC article.
-
Identification of amino acids that are selectively involved in Gi/o activation by rat melanin-concentrating hormone receptor 1.Cell Signal. 2015 Apr;27(4):818-27. doi: 10.1016/j.cellsig.2015.01.008. Epub 2015 Jan 22. Cell Signal. 2015. PMID: 25617691
-
Specific pharmacological and Gi/o protein responses of some native GPCRs in neurons.Nat Commun. 2024 Mar 5;15(1):1990. doi: 10.1038/s41467-024-46177-z. Nat Commun. 2024. PMID: 38443355 Free PMC article.
-
Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling.Pharmacol Rev. 2001 Mar;53(1):1-24. Pharmacol Rev. 2001. PMID: 11171937 Review.
-
G protein-coupled receptor systems and their lipid environment in health disorders during aging.Biochim Biophys Acta. 2007 Apr;1768(4):964-75. doi: 10.1016/j.bbamem.2006.09.024. Epub 2006 Oct 3. Biochim Biophys Acta. 2007. PMID: 17070497 Review.
Cited by
-
Estrogen Receptor and Vascular Aging.Front Aging. 2021 Sep 24;2:727380. doi: 10.3389/fragi.2021.727380. eCollection 2021. Front Aging. 2021. PMID: 35821994 Free PMC article. Review.
-
The Ion Channel and GPCR Toolkit of Brain Capillary Pericytes.Front Cell Neurosci. 2020 Dec 18;14:601324. doi: 10.3389/fncel.2020.601324. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33390906 Free PMC article. Review.
-
Age-dependent effects of oxytocin in brain regions enriched with oxytocin receptors.Psychoneuroendocrinology. 2024 Feb;160:106666. doi: 10.1016/j.psyneuen.2023.106666. Epub 2023 Nov 3. Psychoneuroendocrinology. 2024. PMID: 37951085 Free PMC article. Clinical Trial.
-
Plasma membrane and brain dysfunction of the old: Do we age from our membranes?Front Cell Dev Biol. 2022 Oct 6;10:1031007. doi: 10.3389/fcell.2022.1031007. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36274849 Free PMC article. Review.
-
Are the Acute Effects of THC Different in Aging Adults?Brain Sci. 2021 May 1;11(5):590. doi: 10.3390/brainsci11050590. Brain Sci. 2021. PMID: 34062795 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

