Is Aberrant Reno-Renal Reflex Control of Blood Pressure a Contributor to Chronic Intermittent Hypoxia-Induced Hypertension?
- PMID: 31105584
- PMCID: PMC6491928
- DOI: 10.3389/fphys.2019.00465
Is Aberrant Reno-Renal Reflex Control of Blood Pressure a Contributor to Chronic Intermittent Hypoxia-Induced Hypertension?
Abstract
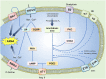
Renal sensory nerves are important in the regulation of body fluid and electrolyte homeostasis, and blood pressure. Activation of renal mechanoreceptor afferents triggers a negative feedback reno-renal reflex that leads to the inhibition of sympathetic nervous outflow. Conversely, activation of renal chemoreceptor afferents elicits reflex sympathoexcitation. Dysregulation of reno-renal reflexes by suppression of the inhibitory reflex and/or activation of the excitatory reflex impairs blood pressure control, predisposing to hypertension. Obstructive sleep apnoea syndrome (OSAS) is causally related to hypertension. Renal denervation in patients with OSAS or in experimental models of chronic intermittent hypoxia (CIH), a cardinal feature of OSAS due to recurrent apnoeas (pauses in breathing), results in a decrease in circulating norepinephrine levels and attenuation of hypertension. The mechanism of the beneficial effect of renal denervation on blood pressure control in models of CIH and OSAS is not fully understood, since renal denervation interrupts renal afferent signaling to the brain and sympathetic efferent signals to the kidneys. Herein, we consider the currently proposed mechanisms involved in the development of hypertension in CIH disease models with a focus on oxidative and inflammatory mediators in the kidneys and their potential influence on renal afferent control of blood pressure, with wider consideration of the evidence available from a variety of hypertension models. We draw focus to the potential contribution of aberrant renal afferent signaling in the development, maintenance and progression of high blood pressure, which may have relevance to CIH-induced hypertension.
Keywords: intermittent hypoxia; neurogenic hypertension; obstructive sleep apnoea syndrome; renal afferent nerves; reno-renal reflexes; sympathoexcitation.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

