Human, Nonhuman Primate, and Bat Cells Are Broadly Susceptible to Tibrovirus Particle Cell Entry
- PMID: 31105663
- PMCID: PMC6499107
- DOI: 10.3389/fmicb.2019.00856
Human, Nonhuman Primate, and Bat Cells Are Broadly Susceptible to Tibrovirus Particle Cell Entry
Abstract
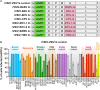
In 2012, the genome of a novel rhabdovirus, Bas-Congo virus (BASV), was discovered in the acute-phase serum of a Congolese patient with presumed viral hemorrhagic fever. In the absence of a replicating virus isolate, fulfilling Koch's postulates to determine whether BASV is indeed a human virus and/or pathogen has been impossible. However, experiments with vesiculoviral particles pseudotyped with Bas-Congo glycoprotein suggested that BASV particles can enter cells from multiple animals, including humans. In 2015, genomes of two related viruses, Ekpoma virus 1 (EKV-1) and Ekpoma virus 2 (EKV-2), were detected in human sera in Nigeria. Isolates could not be obtained. Phylogenetic analyses led to the classification of BASV, EKV-1, and EKV-2 in the same genus, Tibrovirus, together with five biting midge-borne rhabdoviruses [i.e., Beatrice Hill virus (BHV), Bivens Arm virus (BAV), Coastal Plains virus (CPV), Sweetwater Branch virus (SWBV), and Tibrogargan virus (TIBV)] not known to infect humans. Using individual recombinant vesiculoviruses expressing the glycoproteins of all eight known tibroviruses and more than 75 cell lines representing different animal species, we demonstrate that the glycoproteins of all tibroviruses can mediate vesiculovirus particle entry into human, bat, nonhuman primate, cotton rat, boa constrictor, and Asian tiger mosquito cells. Using four of five isolated authentic tibroviruses (i.e., BAV, CPV, SWBV, and TIBV), our experiments indicate that many cell types may be partially resistant to tibrovirus replication after virion cell entry. Consequently, experimental data solely obtained from experiments using tibrovirus surrogate systems (e.g., vesiculoviral pseudotypes, recombinant vesiculoviruses) cannot be used to predict whether BASV, or any other tibrovirus, infects humans.
Keywords: Bas-Congo virus; Mononegavirales; Rhabdoviridae; mononegavirus; rhabdovirus; tibrovirus; tropism; viral hemorrhagic fever.
Figures

References
-
- Altstiel L. D., Landsberger F. R. (1981). Lipid-protein interactions between the surface glycoprotein of vesicular stomatitis virus and the lipid bilayer. Virology 115 1–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

