Effect of Quercetin Rich Onion Extracts on Bacterial Quorum Sensing
- PMID: 31105665
- PMCID: PMC6492534
- DOI: 10.3389/fmicb.2019.00867
Effect of Quercetin Rich Onion Extracts on Bacterial Quorum Sensing
Abstract
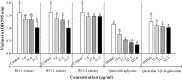
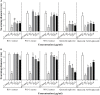
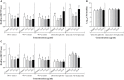
Quorum sensing (QS) regulates bacterial gene expression and studies suggest quercetin, a flavonol found in onion, as a QS inhibitor. There are no studies showing the anti-QS activity of plants containing quercetin in its native glycosylated forms. This study aimed to evaluate the antimicrobial and anti-QS potential of organic extracts of onion varieties and its representative phenolic compounds quercetin aglycone and quercetin 3-β-D-glucoside in the QS model bacteria Chromobacterium violaceum ATCC 12472, Pseudomonas aeruginosa PAO1, and Serratia marcescens MG1. Three phenolic extracts were obtained: red onion extract in methanol acidified with 2.5% acetic acid (RO-1), white onion extract in methanol (WO-1) and white onion extract in methanol ammonium (WO-2). Quercetin 4-O-glucoside and quercetin 3,4-O-diglucoside were identified as the predominant compounds in both onion varieties using HPLC-DAD and LC-ESI-MS/MS. However, quercetin aglycone, cyanidin 3-O-glucoside and quercetin glycoside were identified only in RO-1. The three extracts showed minimum inhibitory concentration (MIC) values equal to or above 125 μg/ml of dried extract. Violacein production was significantly reduced by RO-1 and quercetin aglycone, but not by quercetin 3-β-D-glucoside. Motility in P. aeruginosa PAO1 was inhibited by RO-1, while WO-2 inhibited S. marcescens MG1 motility only in high concentration. Quercetin aglycone and quercetin 3-β-D-glucoside were effective at inhibiting motility in P. aeruginosa PAO1 and S. marcescens MG1. Surprisingly, biofilm formation was not affected by any extracts or the quercetins tested at sub-MIC concentrations. In silico studies suggested a better interaction and placement of quercetin aglycone in the structures of the CviR protein of C. violaceum ATCC 12472 than the glycosylated compound which corroborates the better inhibitory effect of the former over violacein production. On the other hand, the two quercetins were well placed in the AHLs binding pockets of the LasR protein of P. aeruginosa PAO1. Overall onion extracts and quercetin presented antimicrobial activity, and interference on QS regulated production of violacein and swarming motility.
Keywords: antimicrobial activity; glycosylation; onion; phenolic compounds; quorum quenching; quorum sensing.
Figures
Similar articles
-
Anti-quorum sensing activity of Psidium guajava L. flavonoids against Chromobacterium violaceum and Pseudomonas aeruginosa PAO1.Microbiol Immunol. 2014 May;58(5):286-93. doi: 10.1111/1348-0421.12150. Microbiol Immunol. 2014. PMID: 24698116
-
Effect of Capsicum Frutescens Extract, Capsaicin, and Luteolin on Quorum Sensing Regulated Phenotypes.J Food Sci. 2019 Jun;84(6):1477-1486. doi: 10.1111/1750-3841.14648. Epub 2019 May 27. J Food Sci. 2019. PMID: 31132155
-
Attenuation of quorum sensing regulated virulence functions and biofilm of pathogenic bacteria by medicinal plant Artemisia annua and its phytoconstituent 1, 8-cineole.Microsc Res Tech. 2024 Jan;87(1):133-148. doi: 10.1002/jemt.24418. Epub 2023 Sep 20. Microsc Res Tech. 2024. PMID: 37728140
-
Antibiofilm, antiquorum sensing and antioxidant activity of secondary metabolites from seeds of Annona senegalensis, Persoon.Microb Pathog. 2020 Jul;144:104191. doi: 10.1016/j.micpath.2020.104191. Epub 2020 Apr 14. Microb Pathog. 2020. PMID: 32298749
-
Onion Peel Ethylacetate Fraction and Its Derived Constituent Quercetin 4'-O-β-D Glucopyranoside Attenuates Quorum Sensing Regulated Virulence and Biofilm Formation.Front Microbiol. 2017 Sep 5;8:1675. doi: 10.3389/fmicb.2017.01675. eCollection 2017. Front Microbiol. 2017. PMID: 28928721 Free PMC article.
Cited by
-
Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle.Molecules. 2022 Apr 12;27(8):2494. doi: 10.3390/molecules27082494. Molecules. 2022. PMID: 35458691 Free PMC article. Review.
-
Antibiofilm and anti-quorum sensing activity of Psidium guajava L. leaf extract: In vitro and in silico approach.PLoS One. 2023 Dec 19;18(12):e0295524. doi: 10.1371/journal.pone.0295524. eCollection 2023. PLoS One. 2023. PMID: 38113217 Free PMC article.
-
Complex Analysis of Vanillin and Syringic Acid as Natural Antimicrobial Agents against Staphylococcus epidermidis Biofilms.Int J Mol Sci. 2022 Feb 5;23(3):1816. doi: 10.3390/ijms23031816. Int J Mol Sci. 2022. PMID: 35163738 Free PMC article.
-
Impact of Quercetin against Salmonella Typhimurium Biofilm Formation on Food-Contact Surfaces and Molecular Mechanism Pattern.Foods. 2022 Mar 28;11(7):977. doi: 10.3390/foods11070977. Foods. 2022. PMID: 35407064 Free PMC article.
-
Furanone and phytol influence metabolic phenotypes regulated by acyl-homoserine lactone in Salmonella.Braz J Microbiol. 2022 Dec;53(4):2133-2144. doi: 10.1007/s42770-022-00809-y. Epub 2022 Aug 10. Braz J Microbiol. 2022. PMID: 35947344 Free PMC article.
References
-
- Almeida F. A., Vargas E. L. G., Carneiro D. G., Pinto U. M., Vanetti M. C. D. (2018). Virtual screening of plant compounds and nonsteroidal anti-inflammatory drugs for inhibition of quorum sensing and biofilm formation in Salmonella. Microb. Pathog. 121 369–388. 10.1016/j.micpath.2018.05.014 - DOI - PubMed
-
- Al-Yousef H. M., Ahmed A. F., Al-Shabib N. A., Laeeq S., Khan R. A., Rehman M. T., et al. (2017). Onion peel ethyl acetate fraction and its derived constituent quercetin 4′-O-β-D glucopyranoside attenuates quorum sensing regulated virulence and biofilm formation. Front. Microbiol. 8:1675 10.3389/fmicb.2017.01675 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

