Anti-pseudomonad Activity of Manuka Honey and Antibiotics in a Specialized ex vivo Model Simulating Cystic Fibrosis Lung Infection
- PMID: 31105667
- PMCID: PMC6491927
- DOI: 10.3389/fmicb.2019.00869
Anti-pseudomonad Activity of Manuka Honey and Antibiotics in a Specialized ex vivo Model Simulating Cystic Fibrosis Lung Infection
Abstract
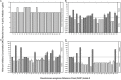
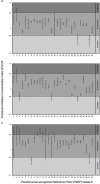
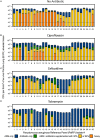
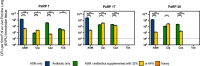
Pseudomonas aeruginosa causes problematic chronic lung infections in those suffering from cystic fibrosis. This is due to its antimicrobial resistance mechanisms and its ability to form robust biofilm communities with increased antimicrobial tolerances. Using novel antimicrobials or repurposing current ones is required in order to overcome these problems. Manuka honey is a natural antimicrobial agent that has been used for many decades in the treatment of chronic surface wounds with great success, particularly those infected with P. aeruginosa. Here we aim to determine whether the antimicrobial activity of manuka honey could potentially be repurposed to inhibit pulmonary P. aeruginosa infections using two ex vivo models. P. aeruginosa isolates (n = 28) from an international panel were tested for their susceptibility to manuka honey and clinically relevant antibiotics (ciprofloxacin, ceftazidime, and tobramycin), alone and in combination, using conventional antimicrobial susceptibility testing (AST). To increase clinical applicability, two ex vivo porcine lung (EVPL) models (using alveolar and bronchiolar tissue) were used to determine the anti-biofilm effects of manuka honey alone and in combination with antibiotics. All P. aeruginosa isolates were susceptible to manuka honey, however, varying incidences of resistance were seen against antibiotics. The combination of sub-inhibitory manuka honey and antibiotics using conventional AST had no effect on activity against the majority of isolates tested. Using the two ex vivo models, 64% (w/v) manuka honey inhibited many of the isolates where abnormally high concentrations of antibiotics could not. Typically, combinations of both manuka honey and antibiotics had increased antimicrobial activity. These results highlight the potential of manuka honey as a future antimicrobial for the treatment of pulmonary P. aeruginosa isolates, clearing potential infection reservoirs within the upper airway.
Keywords: Pseudomonas aeruginosa; antimicrobial susceptibility testing; biofilms; cystic fibrosis; ex vivo model; manuka honey.
Figures

Similar articles
-
Manuka honey treatment of biofilms of Pseudomonas aeruginosa results in the emergence of isolates with increased honey resistance.Ann Clin Microbiol Antimicrob. 2014 May 12;13:19. doi: 10.1186/1476-0711-13-19. Ann Clin Microbiol Antimicrob. 2014. PMID: 24884949 Free PMC article.
-
A demonstration of the susceptibility of clinical isolates obtained from cystic fibrosis patients to manuka honey.Arch Microbiol. 2015 May;197(4):597-601. doi: 10.1007/s00203-015-1091-6. Epub 2015 Feb 14. Arch Microbiol. 2015. PMID: 25680398 Free PMC article.
-
Rifampicin-Manuka Honey Combinations Are Superior to Other Antibiotic-Manuka Honey Combinations in Eradicating Staphylococcus aureus Biofilms.Front Microbiol. 2018 Jan 11;8:2653. doi: 10.3389/fmicb.2017.02653. eCollection 2017. Front Microbiol. 2018. PMID: 29375518 Free PMC article.
-
Combination antimicrobial susceptibility testing for acute exacerbations in chronic infection of Pseudomonas aeruginosa in cystic fibrosis.Cochrane Database Syst Rev. 2020 May 15;5(5):CD006961. doi: 10.1002/14651858.CD006961.pub5. Cochrane Database Syst Rev. 2020. PMID: 32412092 Free PMC article.
-
Combination antimicrobial susceptibility testing for acute exacerbations in chronic infection of Pseudomonas aeruginosa in cystic fibrosis.Cochrane Database Syst Rev. 2017 Jun 19;6(6):CD006961. doi: 10.1002/14651858.CD006961.pub4. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 May 15;5:CD006961. doi: 10.1002/14651858.CD006961.pub5. PMID: 28628280 Free PMC article. Updated. Review.
Cited by
-
Manuka honey in combination with azithromycin shows potential for improved activity against Mycobacterium abscessus.Cell Surf. 2022 Nov 17;8:100090. doi: 10.1016/j.tcsw.2022.100090. eCollection 2022 Dec. Cell Surf. 2022. PMID: 36452962 Free PMC article.
-
Cystic Fibrosis: Recent Insights into Inhaled Antibiotic Treatment and Future Perspectives.Antibiotics (Basel). 2021 Mar 22;10(3):338. doi: 10.3390/antibiotics10030338. Antibiotics (Basel). 2021. PMID: 33810116 Free PMC article. Review.
-
A new model of endotracheal tube biofilm identifies combinations of matrix-degrading enzymes and antimicrobials able to eradicate biofilms of pathogens that cause ventilator-associated pneumonia.Microbiology (Reading). 2024 Aug;170(8):001480. doi: 10.1099/mic.0.001480. Microbiology (Reading). 2024. PMID: 39088248 Free PMC article.
-
Diversity of Monofloral Honey Based on the Antimicrobial and Antioxidant Potential.Antibiotics (Basel). 2022 Apr 28;11(5):595. doi: 10.3390/antibiotics11050595. Antibiotics (Basel). 2022. PMID: 35625239 Free PMC article.
-
Prospects of honey in fighting against COVID-19: pharmacological insights and therapeutic promises.Heliyon. 2020 Dec;6(12):e05798. doi: 10.1016/j.heliyon.2020.e05798. Epub 2020 Dec 21. Heliyon. 2020. PMID: 33363261 Free PMC article. Review.
References
-
- Cystic Fibrosis Trust (2009). Antibiotic Treatment for Cystic Fibrosis 3rd Edn. London: Cystic Fibrosis Trust.
LinkOut - more resources
Full Text Sources

