In vitro Susceptibility and Evaluation of Techniques for Understanding the Mode of Action of a Promising Non-antibiotic Citrus Fruit Extract Against Several Pathogens
- PMID: 31105673
- PMCID: PMC6491944
- DOI: 10.3389/fmicb.2019.00884
In vitro Susceptibility and Evaluation of Techniques for Understanding the Mode of Action of a Promising Non-antibiotic Citrus Fruit Extract Against Several Pathogens
Abstract
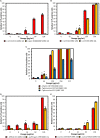
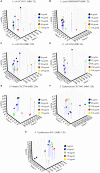
The screening for alternatives to antibiotics is an urgent need for the pharmaceutical industry. One of these alternatives seems to be the citrus fruit extracts, which are showing a significant antibacterial activity against Gram-negative and Gram-positive bacteria. One of these citrus extracts, named BIOCITRO®, is assessed in this study to elucidate its bacteriostatic and bactericidal effect and its mode of action on the important pathogens Campylobacter coli, C. jejuni, Escherichia coli, Salmonella enterica ssp. enterica, Clostridium difficile, C. perfringens, and Staphylococcus aureus. For most of the strains tested of these bacteria the product was bactericidal as well as bacteriostatic at the same concentration, and the minimum bactericidal concentrations ranged from 16 to 256 μg/mL. Regarding the mode of action, important changes in the permeability, structure, composition and morphology of the bacterial envelope were evidenced using flow cytometry, Fourier transform infrared spectroscopy and scanning electron microscopy. The main effect of the product was found over carbohydrates and polysaccharides, inducing the release of microvesicles by the cells in addition to other specific effects. During the study, the techniques used were evaluated to clarify their contribution to the knowledge of the mode of action of the product. The survival test elucidated whether the modifications displayed using other techniques affected the viability of the cells or on the contrary, the cells remained viable even with evident changes in their structure, composition or morphology. Flow cytometry showed that for some strains the proportion of cells detected with altered membrane permeability were higher than the number of non-viable cells, and therefore the damage did not affect the viability of some cells. On the contrary, some cells observed using scanning electron microscopy with no apparent damage, were demonstrated non-viable using the survival test, making this technique indispensable in studies of the mode of action of antimicrobials to make a correct interpretation of the data from other techniques.
Keywords: BIOCITRO; alternative to antibiotics; antibacterial; antimicrobial; mode of action; phytobiotics; plant extracts; susceptibility.
Figures





Similar articles
-
In-depth in vitro Evaluation of the Activity and Mechanisms of Action of Organic Acids and Essential Oils Against Swine Enteropathogenic Bacteria.Front Vet Sci. 2020 Nov 10;7:572947. doi: 10.3389/fvets.2020.572947. eCollection 2020. Front Vet Sci. 2020. PMID: 33240953 Free PMC article.
-
In vitro susceptibility of Brachyspira hyodysenteriae to a commercial citrus fruit extract.Res Vet Sci. 2017 Dec;115:318-324. doi: 10.1016/j.rvsc.2017.06.010. Epub 2017 Jun 20. Res Vet Sci. 2017. PMID: 28651094
-
Antibacterial activity and mode of action of a commercial citrus fruit extract.J Appl Microbiol. 2013 Jul;115(1):50-60. doi: 10.1111/jam.12216. Epub 2013 May 3. J Appl Microbiol. 2013. PMID: 23581704
-
Exploiting fruit byproducts for eco-friendly nanosynthesis: Citrus × clementina peel extract mediated fabrication of silver nanoparticles with high efficacy against microbial pathogens and rat glial tumor C6 cells.Environ Sci Pollut Res Int. 2018 Apr;25(11):10250-10263. doi: 10.1007/s11356-017-8724-z. Epub 2017 Mar 17. Environ Sci Pollut Res Int. 2018. PMID: 28303540
-
Cutting-edge knowledge on the roles of phytobiotics and their proposed modes of action in swine.Front Vet Sci. 2023 Sep 20;10:1265689. doi: 10.3389/fvets.2023.1265689. eCollection 2023. Front Vet Sci. 2023. PMID: 37808106 Free PMC article. Review.
Cited by
-
Broad-Spectrum Antimicrobial Activity of Oftasecur and Visuprime Ophthalmic Solutions.Microorganisms. 2023 Feb 17;11(2):503. doi: 10.3390/microorganisms11020503. Microorganisms. 2023. PMID: 36838468 Free PMC article.
-
In vitro Assessment of Antiviral Effect of Natural Compounds on Porcine Epidemic Diarrhea Coronavirus.Front Vet Sci. 2021 Mar 29;8:652000. doi: 10.3389/fvets.2021.652000. eCollection 2021. Front Vet Sci. 2021. PMID: 33855058 Free PMC article.
-
Exopolysaccharide from the yeast Papiliotrema terrestris PT22AV for skin wound healing.J Adv Res. 2023 Apr;46:61-74. doi: 10.1016/j.jare.2022.06.012. Epub 2022 Jun 25. J Adv Res. 2023. PMID: 35760297 Free PMC article.
-
Frontiers in antibiotic alternatives for Clostridioides difficile infection.World J Gastroenterol. 2021 Nov 14;27(42):7210-7232. doi: 10.3748/wjg.v27.i42.7210. World J Gastroenterol. 2021. PMID: 34876784 Free PMC article.
-
In-depth in vitro Evaluation of the Activity and Mechanisms of Action of Organic Acids and Essential Oils Against Swine Enteropathogenic Bacteria.Front Vet Sci. 2020 Nov 10;7:572947. doi: 10.3389/fvets.2020.572947. eCollection 2020. Front Vet Sci. 2020. PMID: 33240953 Free PMC article.
References
-
- Alvarez-Ordóñez A., Prieto M. (2012). Fourier Transform Infrared Spectroscopy in Food Microbiology. Boston, MA: Springer.
LinkOut - more resources
Full Text Sources

