PD-1 Dynamically Regulates Inflammation and Development of Brain-Resident Memory CD8 T Cells During Persistent Viral Encephalitis
- PMID: 31105690
- PMCID: PMC6499176
- DOI: 10.3389/fimmu.2019.00783
PD-1 Dynamically Regulates Inflammation and Development of Brain-Resident Memory CD8 T Cells During Persistent Viral Encephalitis
Abstract
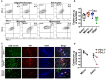
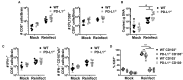
Programmed cell death-1 (PD-1) receptor signaling dampens the functionality of T cells faced with repetitive antigenic stimulation from chronic infections or tumors. Using intracerebral (i.c.) inoculation with mouse polyomavirus (MuPyV), we have shown that CD8 T cells establish a PD-1hi, tissue-resident memory population in the brains (bTRM) of mice with a low-level persistent infection. In MuPyV encephalitis, PD-L1 was expressed on infiltrating myeloid cells, microglia and astrocytes, but not on oligodendrocytes. Engagement of PD-1 on anti-MuPyV CD8 T cells limited their effector activity. NanoString gene expression analysis showed that neuroinflammation was higher in PD-L1-/- than wild type mice at day 8 post-infection, the peak of the MuPyV-specific CD8 response. During the persistent phase of infection, however, the absence of PD-1 signaling was found to be associated with a lower inflammatory response than in wild type mice. Genetic disruption and intracerebroventricular blockade of PD-1 signaling resulted in an increase in number of MuPyV-specific CD8 bTRM and the fraction of these cells expressing CD103, the αE integrin commonly used to define tissue-resident T cells. However, PD-L1-/- mice persistently infected with MuPyV showed impaired virus control upon i.c. re-infection with MuPyV. Collectively, these data reveal a temporal duality in PD-1-mediated regulation of MuPyV-associated neuroinflammation. PD-1 signaling limited the severity of neuroinflammation during acute infection but sustained a level of inflammation during persistent infection for maintaining control of virus re-infection.
Keywords: CD8 T cells; PD-1; PD-L1; mouse polyomavirus; neuroinflammation; tissue-resident memory; viral encephalitis.
Figures

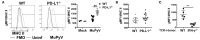



Similar articles
-
Maintenance of PD-1 on brain-resident memory CD8 T cells is antigen independent.Immunol Cell Biol. 2017 Nov;95(10):953-959. doi: 10.1038/icb.2017.62. Epub 2017 Aug 22. Immunol Cell Biol. 2017. PMID: 28829048 Free PMC article.
-
CD8 T Cells and STAT1 Signaling Are Essential Codeterminants in Protection from Polyomavirus Encephalopathy.J Virol. 2020 Mar 31;94(8):e02038-19. doi: 10.1128/JVI.02038-19. Print 2020 Mar 31. J Virol. 2020. PMID: 31996425 Free PMC article.
-
The PD-1: PD-L1 pathway promotes development of brain-resident memory T cells following acute viral encephalitis.J Neuroinflammation. 2017 Apr 13;14(1):82. doi: 10.1186/s12974-017-0860-3. J Neuroinflammation. 2017. PMID: 28407741 Free PMC article.
-
Brain-Resident T Cells Following Viral Infection.Viral Immunol. 2019 Jan/Feb;32(1):48-54. doi: 10.1089/vim.2018.0084. Epub 2018 Sep 18. Viral Immunol. 2019. PMID: 30230418 Free PMC article. Review.
-
Gamma-Chain Receptor Cytokines & PD-1 Manipulation to Restore HCV-Specific CD8+ T Cell Response during Chronic Hepatitis C.Cells. 2021 Mar 3;10(3):538. doi: 10.3390/cells10030538. Cells. 2021. PMID: 33802622 Free PMC article. Review.
Cited by
-
A case report of the diagnosis and treatment of immune checkpoint inhibitor-related encephalitis induced by camrelizumab.AME Case Rep. 2024 Sep 6;8:101. doi: 10.21037/acr-24-58. eCollection 2024. AME Case Rep. 2024. PMID: 39380870 Free PMC article.
-
To Go or Stay: The Development, Benefit, and Detriment of Tissue-Resident Memory CD8 T Cells during Central Nervous System Viral Infections.Viruses. 2019 Sep 11;11(9):842. doi: 10.3390/v11090842. Viruses. 2019. PMID: 31514273 Free PMC article. Review.
-
Transcriptomic and clonal characterization of T cells in the human central nervous system.Sci Immunol. 2020 Sep 18;5(51):eabb8786. doi: 10.1126/sciimmunol.abb8786. Sci Immunol. 2020. PMID: 32948672 Free PMC article.
-
Tissue-resident immune cells in the pathogenesis of multiple sclerosis.Inflamm Res. 2023 Mar;72(3):363-372. doi: 10.1007/s00011-022-01677-w. Epub 2022 Dec 22. Inflamm Res. 2023. PMID: 36547688 Review.
-
Programmed death ligand-1 induction restrains the cytotoxic T lymphocyte response against microglia.Glia. 2021 Apr;69(4):858-871. doi: 10.1002/glia.23932. Epub 2020 Oct 31. Glia. 2021. PMID: 33128485 Free PMC article.
References
-
- Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. (2004) 173:945–54. 10.4049/jimmunol.173.2.945 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

