The Axial Organ and the Pharynx Are Sites of Hematopoiesis in the Sea Urchin
- PMID: 31105697
- PMCID: PMC6494969
- DOI: 10.3389/fimmu.2019.00870
The Axial Organ and the Pharynx Are Sites of Hematopoiesis in the Sea Urchin
Abstract
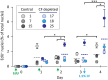
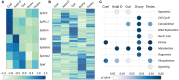
Background: The location of coelomocyte proliferation in adult sea urchins is unknown and speculations since the early 1800s have been based on microanatomy and tracer uptake studies. In adult sea urchins (Strongylocentrotus purpuratus) with down-regulated immune systems, coelomocyte numbers increase in response to immune challenge, and whether some or all of these cells are newly proliferated is not known. The gene regulatory network that encodes transcription factors that control hematopoiesis in embryonic and larval sea urchins has not been investigated in adults. Hence, to identify the hematopoietic tissue in adult sea urchins, cell proliferation, expression of phagocyte specific genes, and expression of genes encoding transcription factors that function in the conserved regulatory network that controls hematopoiesis in embryonic and larval sea urchins were investigated for several tissues. Results: Cell proliferation was induced in adult sea urchins either by immune challenge through injection of heat-killed Vibrio diazotrophicus or by cell depletion through aspiration of coelomic fluid. In response to either of these stimuli, newly proliferated coelomocytes constitute only about 10% of the cells in the coelomic fluid. In tissues, newly proliferated cells and cells that express SpTransformer proteins (formerly Sp185/333) that are markers for phagocytes are present in the axial organ, gonad, pharynx, esophagus, and gut with no differences among tissues. The expression level of genes encoding transcription factors that regulate hematopoiesis show that both the axial organ and the pharynx have elevated expression compared to coelomocytes, esophagus, gut, and gonad. Similarly, an RNAseq dataset shows similar results for the axial organ and pharynx, but also suggests that the axial organ may be a site for removal and recycling of cells in the coelomic cavity. Conclusions: Results presented here are consistent with previous speculations that the axial organ may be a site of coelomocyte proliferation and that it may also be a center for cellular removal and recycling. A second site, the pharynx, may also have hematopoietic activity, a tissue that has been assumed to function only as part of the intestinal tract.
Keywords: EdU; echinoderm; gene regulatory network; hematopoietic tissue; proliferation; transcription factors.
Figures





Similar articles
-
Coelomocyte populations in the sea urchin, Strongylocentrotus purpuratus, undergo dynamic changes in response to immune challenge.Front Immunol. 2022 Aug 31;13:940852. doi: 10.3389/fimmu.2022.940852. eCollection 2022. Front Immunol. 2022. PMID: 36119116 Free PMC article.
-
The Sp185/333 immune response genes and proteins are expressed in cells dispersed within all major organs of the adult purple sea urchin.Innate Immun. 2013 Dec;19(6):569-87. doi: 10.1177/1753425912473850. Epub 2013 Feb 12. Innate Immun. 2013. PMID: 23405032
-
Single sea urchin phagocytes express messages of a single sequence from the diverse Sp185/333 gene family in response to bacterial challenge.J Immunol. 2014 Dec 1;193(11):5678-88. doi: 10.4049/jimmunol.1401681. Epub 2014 Oct 29. J Immunol. 2014. PMID: 25355922
-
Methods for collection, handling, and analysis of sea urchin coelomocytes.Methods Cell Biol. 2019;150:357-389. doi: 10.1016/bs.mcb.2018.11.009. Epub 2019 Jan 9. Methods Cell Biol. 2019. PMID: 30777184 Review.
-
The SpTransformer Gene Family (Formerly Sp185/333) in the Purple Sea Urchin and the Functional Diversity of the Anti-Pathogen rSpTransformer-E1 Protein.Front Immunol. 2017 Jun 30;8:725. doi: 10.3389/fimmu.2017.00725. eCollection 2017. Front Immunol. 2017. PMID: 28713368 Free PMC article. Review.
Cited by
-
Recombinant SpTransformer proteins bind to specific sites on sea urchin phagocytes and modulate SpTransformer gene expression and immune responsiveness.Front Immunol. 2025 Jan 28;15:1496832. doi: 10.3389/fimmu.2024.1496832. eCollection 2024. Front Immunol. 2025. PMID: 39936151 Free PMC article.
-
Recombinant SpTransformer proteins are functionally diverse for binding and phagocytosis by three subtypes of sea urchin phagocytes.Front Immunol. 2024 Apr 29;15:1372904. doi: 10.3389/fimmu.2024.1372904. eCollection 2024. Front Immunol. 2024. PMID: 38742116 Free PMC article.
-
Regulation of dynamic pigment cell states at single-cell resolution.Elife. 2020 Aug 19;9:e60388. doi: 10.7554/eLife.60388. Elife. 2020. PMID: 32812865 Free PMC article.
-
Widespread presence of bone marrow-like hematopoietic stem cell niche in invertebrate skeletons.Sci Adv. 2025 Jun 27;11(26):eadw0958. doi: 10.1126/sciadv.adw0958. Epub 2025 Jun 25. Sci Adv. 2025. PMID: 40561015 Free PMC article.
-
Immunomodulatory Function of Polyvinylpyrrolidone (PVP)-Functionalized Gold Nanoparticles in Vibrio-Stimulated Sea Urchin Immune Cells.Nanomaterials (Basel). 2021 Oct 8;11(10):2646. doi: 10.3390/nano11102646. Nanomaterials (Basel). 2021. PMID: 34685085 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

