Ocular gene therapy for choroideremia: clinical trials and future perspectives
- PMID: 31105764
- PMCID: PMC6520227
- DOI: 10.1080/17469899.2018.1475232
Ocular gene therapy for choroideremia: clinical trials and future perspectives
Abstract
Introduction: Gene therapy offers the potential for targeted replacement of single gene defects in inherited retinal degenerations.
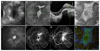
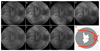
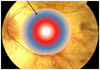
Areas covered: Choroideremia is an X-linked blinding retinal disease resulting from deficiency of the CHM gene product, REP1. The disease represents an ideal target for retinal gene therapy, as it is readily diagnosed in the clinic, relatively homogenous in phenotype and slow progressing, thereby providing a wide therapeutic window for intervention. Ongoing clinical trials of retinal gene therapy for choroideremia using an adeno-associated viral vector have demonstrated safety and early efficacy. We review the clinical characteristics of the disease with a view to interpreting the findings of gene therapy clinical trials and discuss future directions.
Expert commentary: Choroideremia gene therapy has so far demonstrated good safety profile and early functional visual acuity gains in a proportion of trial participants, which appear to be sustained.
Keywords: AAV; CHM; REP1; adeno-associated virus; choroideremia; gene therapy.
Conflict of interest statement
Declaration of interest RE MacLaren is the scientific founder of Nightstar Therapeutics Inc. RE MacLaren is a consultant to Spark Therapeutics Inc. Neither companies had any role in the writing of this review article. The views expressed are those of the authors and not necessarily those of the Wellcome Trust, the National Health Service, the NIHR, or the UK Department of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. One peer reviewer was a scientific director of a trial being run by Spark Therapeutics.
Figures
References
-
- Cremers FP, van de Pol DJ, van Kerkhoff LP, et al. Cloning of a gene that is rearranged in patients with choroideraemia. Nature. 1990;347:674–677. [•• Identification of the gene underlying choroideremia.] - PubMed
-
- Seabra MC, Brown MS, Goldstein JL. Retinal degeneration in choroideremia: deficiency of rab geranylgeranyl transferase. Science. 1993;259:377–381. [•• Identification of prenylation defect in choroideremia.] - PubMed
-
- van Bokhoven H, van den Hurk JAJM, Bogerd LPM, et al. Cloning and characterization of the human choroideremia gene. Hum Mol Genet. 1994;3:1041–1046. - PubMed
-
- van den Hurk JA, Schwartz M, van Bokhoven H, et al. Molecular basis of choroideremia (CHM): mutations involving the Rab escort protein-1 (REP-1) gene. Hum Mutat. 1997;9:110–117. Review. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
