Dose-related histopathology and bone remodeling characteristics of the knee articular cartilage and subchondral bone induced by glucocorticoids in rats
- PMID: 31105787
- PMCID: PMC6507510
- DOI: 10.3892/etm.2019.7508
Dose-related histopathology and bone remodeling characteristics of the knee articular cartilage and subchondral bone induced by glucocorticoids in rats
Abstract
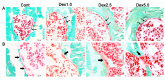
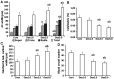
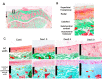
The aim of the current study was to investigate histopathological changes and bone remodeling in the knee articular cartilage and subchondral bone in rats following treatment with glucocorticoids. A total of 30 3-month-old female Sprague-Dawley rats were randomly divided into either a vehicle control group or one of three experimental groups wherein dexamethasone (Dex) was administered at a dose of 1.0, 2.5 or 5.0 mg/kg (Dex1.0, Dex2.5 and Dex5.0, respectively), for 8 weeks. Articular cartilage and the epiphyseal subchondral bone of the proximal tibias were evaluated by histopathology or for bone remodeling using histomorphometry. No histological changes were identified in the knee articular cartilage but the bone formation rate of the subchondral bone was lower in the Dex1.0 group compared with that of the control group. Compared with the control and the Dex1.0 group, the width of the articular cartilage and the subchondral plate were larger, with abnormal morphology and increased apoptosis of chondrocytes, decreased cell/matrix volume ratio in the cartilage and fewer blood vessels in the subchondral plate in the Dex2.5 and Dex5.0 groups. A higher Dex dose resulted in more severe inhibition of bone formation, a greater number of apoptotic osteocytes and constrained bone resorption. All microstructure parameters indicated no significant changes in the Dex2.5 group but exhibited deterioration in the Dex5.0 group compared with the normal and Dex1.0 group. There were no significant differences in morphological changes, or in static and dynamic bone indices between the Dex2.5 and Dex5.0 groups. In conclusion, long-term glucocorticoid use induced dose-related histopathological changes in the knee articular cartilage, along with unbalanced bone remodeling and osteopenia in the subchondral bone. The degree of damage to the articular cartilage was milder and transformed from compensation to degeneration at higher doses.
Keywords: articular cartilage; glucocorticoid; knee; rat; subchondral bone.
Figures

Similar articles
-
Protective effect of zoledronic acid on articular cartilage and subchondral bone of rabbits with experimental knee osteoarthritis.Exp Ther Med. 2017 Nov;14(5):4901-4909. doi: 10.3892/etm.2017.5135. Epub 2017 Sep 19. Exp Ther Med. 2017. PMID: 29201194 Free PMC article.
-
Changes in articular cartilage and subchondral bone histomorphometry in osteoarthritic knee joints in humans.Bone. 2003 Mar;32(3):284-90. doi: 10.1016/s8756-3282(02)00982-1. Bone. 2003. PMID: 12667556
-
Control of Dkk-1 ameliorates chondrocyte apoptosis, cartilage destruction, and subchondral bone deterioration in osteoarthritic knees.Arthritis Rheum. 2010 May;62(5):1393-402. doi: 10.1002/art.27357. Arthritis Rheum. 2010. PMID: 20131282
-
Spatial and temporal changes of subchondral bone proceed to articular cartilage degeneration in rats subjected to knee immobilization.Microsc Res Tech. 2016 Mar;79(3):209-18. doi: 10.1002/jemt.22620. Epub 2016 Jan 11. Microsc Res Tech. 2016. PMID: 26910643
-
Properties of Cartilage-Subchondral Bone Junctions: A Narrative Review with Specific Focus on the Growth Plate.Cartilage. 2021 Dec;13(2_suppl):16S-33S. doi: 10.1177/1947603520924776. Epub 2020 May 27. Cartilage. 2021. PMID: 32458695 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
