Genomic landscape analyses of reprogrammed cells using integrative and non-integrative methods reveal variable cancer-associated alterations
- PMID: 31105870
- PMCID: PMC6505633
- DOI: 10.18632/oncotarget.26857
Genomic landscape analyses of reprogrammed cells using integrative and non-integrative methods reveal variable cancer-associated alterations
Abstract
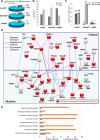
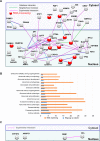
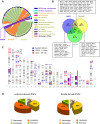
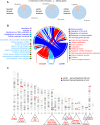
Recent development of cell reprogramming technologies brought a major hope for future cell therapy applications by the use of these cells or their derivatives. For this purpose, one of the major requirements is the absence of genomic alterations generating a risk of cell transformation. Here we analyzed by microarray-based comparative genomic hybridization human iPSC generated by two non-integrative and one integrative method at pluripotent stage as well as in corresponding teratomas. We show that all iPSC lines exhibit copy number variations (CNV) of several genes deregulated in oncogenesis. These cancer-associated genomic alterations were more pronounced in virally programmed hiPSCs and their derivative teratoma as compared to those found in iPSC generated by mRNA-mediated reprogramming. Bioinformatics analysis showed the involvement of these genes in human leukemia and carcinoma. We conclude that genetic screening should become a standard procedure to ensure that hiPSCs are free from cancer-associated genomic alterations before clinical use.
Keywords: CGH array; genetic instability; iPSCs.
Conflict of interest statement
CONFLICTS OF INTEREST There are no conflicts of interest to disclose.
Figures

Similar articles
-
Effects of Integrating and Non-Integrating Reprogramming Methods on Copy Number Variation and Genomic Stability of Human Induced Pluripotent Stem Cells.PLoS One. 2015 Jul 1;10(7):e0131128. doi: 10.1371/journal.pone.0131128. eCollection 2015. PLoS One. 2015. PMID: 26131765 Free PMC article.
-
Robust Differentiation of mRNA-Reprogrammed Human Induced Pluripotent Stem Cells Toward a Retinal Lineage.Stem Cells Transl Med. 2016 Apr;5(4):417-26. doi: 10.5966/sctm.2015-0093. Epub 2016 Mar 1. Stem Cells Transl Med. 2016. PMID: 26933039 Free PMC article.
-
Methods of Reprogramming to Induced Pluripotent Stem Cell Associated with Chromosomal Integrity and Delineation of a Chromosome 5q Candidate Region for Growth Advantage.Stem Cells Dev. 2015 Sep 1;24(17):2032-40. doi: 10.1089/scd.2015.0061. Epub 2015 May 18. Stem Cells Dev. 2015. PMID: 25867454
-
[Analysis of genomic copy number alterations of malignant lymphomas and its application for diagnosis].Gan To Kagaku Ryoho. 2007 Jul;34(7):975-82. Gan To Kagaku Ryoho. 2007. PMID: 17637530 Review. Japanese.
-
Genome stability of programmed stem cell products.Adv Drug Deliv Rev. 2017 Oct 1;120:108-117. doi: 10.1016/j.addr.2017.09.004. Epub 2017 Sep 13. Adv Drug Deliv Rev. 2017. PMID: 28917518 Review.
Cited by
-
[Human cells for therapeutics purpose: State of the art].Bull Acad Natl Med. 2020 Oct;204(8):866-876. doi: 10.1016/j.banm.2020.07.051. Epub 2020 Jul 21. Bull Acad Natl Med. 2020. PMID: 32836290 Free PMC article. Review. French.
-
Deciphering the tumour microenvironment of clear cell renal cell carcinoma: Prognostic insights from programmed death genes using machine learning.J Cell Mol Med. 2024 Jul;28(13):e18524. doi: 10.1111/jcmm.18524. J Cell Mol Med. 2024. PMID: 39011666 Free PMC article.
-
Current Landscape of iPSC Haplobanks.Stem Cell Rev Rep. 2024 Nov;20(8):2155-2164. doi: 10.1007/s12015-024-10783-7. Epub 2024 Sep 14. Stem Cell Rev Rep. 2024. PMID: 39276260 Free PMC article. Review.
References
-
- Hussein SM, Batada NN, Vuoristo S, Ching RW, Autio R, Närvä E, Ng S, Sourour M, Hämäläinen R, Olsson C, Lundin K, Mikkola M, Trokovic R, et al. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471:58–62. - PubMed
LinkOut - more resources
Full Text Sources

