Cost-effectiveness analysis of using onasemnogene abeparvocec (AVXS-101) in spinal muscular atrophy type 1 patients
- PMID: 31105909
- PMCID: PMC6508058
- DOI: 10.1080/20016689.2019.1601484
Cost-effectiveness analysis of using onasemnogene abeparvocec (AVXS-101) in spinal muscular atrophy type 1 patients
Abstract
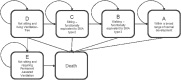
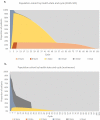
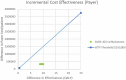
Background: Spinal muscular atrophy type 1 (SMA1) is a devastating genetic disease for which gene-replacement therapy may bring substantial survival and quality of life benefits. Objective: This study investigated the cost-effectiveness of onasemnogene abeparvovec (AVXS-101) gene-replacement therapy for SMA1. Study design: A Markov model was used to estimate the incremental cost-effectiveness ratio (ICER), expressed as cost/quality-adjusted life year ($/QALY), of AVXS-101 versus nusinersen over a lifetime. Survival, healthcare costs and QALYs were estimated using natural history data for SMA patients who achieved motor milestones (sitting/walking). Health utility weights were obtained from the CHERISH trial. Setting: USA; commercial payer perspective Participants: SMA1 infants Interventions: AVXS-101 was compared to nusinersen. Main outcome measure: The primary outcome was the ICER. Results: Expected survival (undiscounted) over a lifetime predicted by the model was 37.20 life years for AVXS-101 and 9.68 for nusinersen (discounted QALYs, 15.65 and 5.29, respectively). Using a potential AVXS-101 price range ($2.5-5.0M/treatment), the average lifetime cost/patient was $4.2-6.6M for AVXS-101 and $6.3M for nusinersen. The ICER range was (-$203,072) to $31,379 per QALY gained for AVXS-101 versus nusinersen, indicating that AVXS-101 was cost-effective with prices of ≤$5M. Conclusion: Single-dose AVXS-101 was cost-effective compared to chronic nusinersen for SMA1 patients.
Keywords: AVXS-101; cost-effectiveness; gene-replacement therapy; nusinersen; onasemnogene abeparvovec; spinal muscular atrophy type 1.
Figures

Similar articles
-
An updated cost-utility model for onasemnogene abeparvovec (Zolgensma®) in spinal muscular atrophy type 1 patients and comparison with evaluation by the Institute for Clinical and Effectiveness Review (ICER).J Mark Access Health Policy. 2021 Feb 28;9(1):1889841. doi: 10.1080/20016689.2021.1889841. J Mark Access Health Policy. 2021. PMID: 33708361 Free PMC article.
-
Early Cost-Effectiveness of Onasemnogene Abeparvovec-xioi (Zolgensma) and Nusinersen (Spinraza) Treatment for Spinal Muscular Atrophy I in The Netherlands With Relapse Scenarios.Value Health. 2021 Jun;24(6):759-769. doi: 10.1016/j.jval.2020.09.021. Epub 2021 Mar 31. Value Health. 2021. PMID: 34119073
-
Survival, Motor Function, and Motor Milestones: Comparison of AVXS-101 Relative to Nusinersen for the Treatment of Infants with Spinal Muscular Atrophy Type 1.Adv Ther. 2019 May;36(5):1164-1176. doi: 10.1007/s12325-019-00923-8. Epub 2019 Mar 16. Adv Ther. 2019. PMID: 30879249 Free PMC article.
-
From Clinical Trials to Clinical Practice: Practical Considerations for Gene Replacement Therapy in SMA Type 1.Pediatr Neurol. 2019 Nov;100:3-11. doi: 10.1016/j.pediatrneurol.2019.06.007. Epub 2019 Jun 13. Pediatr Neurol. 2019. PMID: 31371124 Review.
-
Matching-adjusted indirect treatment comparison of onasemnogene abeparvovec and nusinersen for the treatment of symptomatic patients with spinal muscular atrophy type 1.Curr Med Res Opin. 2021 Oct;37(10):1719-1730. doi: 10.1080/03007995.2021.1947216. Epub 2021 Jul 20. Curr Med Res Opin. 2021. PMID: 34236007
Cited by
-
Costs of Illness of Spinal Muscular Atrophy: A Systematic Review.Appl Health Econ Health Policy. 2021 Jul;19(4):501-520. doi: 10.1007/s40258-020-00624-2. Epub 2021 Feb 12. Appl Health Econ Health Policy. 2021. PMID: 33576939 Free PMC article.
-
Pharmacoeconomic Profiles of Advanced Therapy Medicinal Products in Rare Diseases: A Systematic Review.Healthcare (Basel). 2025 Aug 2;13(15):1894. doi: 10.3390/healthcare13151894. Healthcare (Basel). 2025. PMID: 40805927 Free PMC article. Review.
-
Restoring Protein Expression in Neuromuscular Conditions: A Review Assessing the Current State of Exon Skipping/Inclusion and Gene Therapies for Duchenne Muscular Dystrophy and Spinal Muscular Atrophy.BioDrugs. 2021 Jul;35(4):389-399. doi: 10.1007/s40259-021-00486-7. Epub 2021 Jun 7. BioDrugs. 2021. PMID: 34097287 Review.
-
Systematic Literature Review to Assess Economic Evaluations in Spinal Muscular Atrophy (SMA).Pharmacoeconomics. 2022 Apr;40(Suppl 1):69-89. doi: 10.1007/s40273-021-01095-6. Epub 2021 Oct 18. Pharmacoeconomics. 2022. PMID: 34658008 Free PMC article.
-
Cost-effectiveness analysis of gene-based therapies for patients with spinal muscular atrophy type I in Australia.J Neurol. 2022 Dec;269(12):6544-6554. doi: 10.1007/s00415-022-11319-0. Epub 2022 Aug 18. J Neurol. 2022. PMID: 35980467 Free PMC article.
References
-
- De Sanctis R, Pane M, Coratti G, et al. Clinical phenotypes and trajectories of disease progression in type 1 spinal muscular atrophy. Neuromuscul Disord. 2018;28(1):24–28. Epub 2017/ 10/10 PubMed PMID: 29174525. - PubMed
LinkOut - more resources
Full Text Sources
