Distribution and colocalization of melatonin 1a-receptor and NADPH-d in the trigeminal system of rat
- PMID: 31106073
- PMCID: PMC6500374
- DOI: 10.7717/peerj.6877
Distribution and colocalization of melatonin 1a-receptor and NADPH-d in the trigeminal system of rat
Abstract
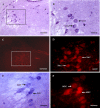
Melatonin and nitric oxide (NO) are involved in orofacial signal processing in the trigeminal sensory system. The aim of the present study was to examine the distribution of melatonin 1a-receptor (MT1) and its colocalization with nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-d) in the spinal trigeminal nucleus (STN), the trigeminal ganglion (TG), and the mesencephalic trigeminal nucleus (MTN) in the rat, using histochemistry and immunohistochemistry. Our results show that MT1-positive neurons are widely distributed in the TG and the subnucleus caudalis of the STN. Furthermore, we found that MT1 colocalizes with NADPH-d throughout the TG and MTN, most extensively in the TG. The distribution pattern of MT1 and its colocalization with NADPH-d indicate that melatonin might play an important role in the trigeminal sensory system, which could be responsible for the regulation of NO levels.
Keywords: Melatonin 1a-receptor; Mesencephalic trieminal nucleus; NADPH-diaphorase; Spinal trigeminal nucleus; Trigeminal ganglion.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Orofacial inflammatory pain affects the expression of MT1 and NADPH-d in rat caudal spinal trigeminal nucleus and trigeminal ganglion.Neural Regen Res. 2013 Nov 15;8(32):2991-3002. doi: 10.3969/j.issn.1673-5374.2013.32.002. Neural Regen Res. 2013. PMID: 25206619 Free PMC article.
-
Distribution of NADPH-diaphorase and nitric oxide synthase in the trigeminal ganglion and mesencephalic trigeminal nucleus of the cat. A histochemical and immunohistochemical study.Acta Anat (Basel). 1998;163(4):191-200. doi: 10.1159/000046498. Acta Anat (Basel). 1998. PMID: 10072567
-
Distribution of heme oxygenase-2 and NADPH-diaphorase in the spinal trigeminal nucleus of the rat.J Mol Histol. 2009 Jun;40(3):209-15. doi: 10.1007/s10735-009-9232-3. Epub 2009 Oct 11. J Mol Histol. 2009. PMID: 19821077
-
Comparative analysis of the chemical neuroanatomy of the mammalian trigeminal ganglion and mesencephalic trigeminal nucleus.Prog Neurobiol. 2002 Jan;66(1):19-59. doi: 10.1016/s0301-0082(01)00021-1. Prog Neurobiol. 2002. PMID: 11897404 Review.
-
Neurobiology of orofacial proprioception.Brain Res Rev. 2007 Dec;56(2):362-83. doi: 10.1016/j.brainresrev.2007.08.009. Epub 2007 Sep 5. Brain Res Rev. 2007. PMID: 17915334 Review.
Cited by
-
Safety and Efficacy of Melatonin in Chronic Tension-Type Headache: A Post-Marketing Real-World Surveillance Program.Pain Ther. 2020 Dec;9(2):741-750. doi: 10.1007/s40122-020-00207-y. Epub 2020 Oct 16. Pain Ther. 2020. PMID: 33067764 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

