A chemical treatment method for obtaining clean and intact pollen shells of different species
- PMID: 31106262
- PMCID: PMC6516503
- DOI: 10.1021/acsbiomaterials.8b00304
A chemical treatment method for obtaining clean and intact pollen shells of different species
Abstract
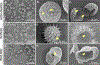
Pollen grains and plant spores have emerged as a novel biomaterial for a broad range of applications including oral drug and vaccine delivery, catalyst support, and removal of heavy metals. However, before pollens can be used, their intrinsic biomolecules, which occupy a large part of the pollen inner cavity must be removed not only to create empty space but because they have potential to cause allergies when used in vivo. These intrinsic materials in the pollen core can be extracted through a chemical treatment to generate clean pollen shells. The commonly used method involves a series of sequential treatments with organic solvents, alkalis, and acids to remove the native pollen biomolecules. This method, though successful for treating lycopodium (Lycopodium clavatum) spores, fails for other species of pollens such as common ragweed (Ambrosia elatior) and thus prevents widespread investigation of different pollens. Herein, we report a new chemical treatment for obtaining clean pollen shells from multiple plant species. This new method involves sequential treatment with acetone, phosphoric acid, and potassium hydroxide. Scanning electron micrographs and protein quantification have shown that the new method can successfully produce clean, intact, and hollow shells from many pollen species including ragweed, sunflower, black alder, and lamb's quarters. These results demonstrate the broad applicability of this method to clean pollens of different species, and paves the way to start investigating them for various applications.
Keywords: Pollen aperture; Pollen treatment; Pollen turgor pressure; Ragweed pollen; Sporopollenin.
Conflict of interest statement
This potential conflict of interest has been disclosed and is managed by Texas Tech University.
Figures









References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
