A four-group urine risk classifier for predicting outcomes in patients with prostate cancer
- PMID: 31106513
- PMCID: PMC6851983
- DOI: 10.1111/bju.14811
A four-group urine risk classifier for predicting outcomes in patients with prostate cancer
Abstract
Objectives: To develop a risk classifier using urine-derived extracellular vesicle (EV)-RNA capable of providing diagnostic information on disease status prior to biopsy, and prognostic information for men on active surveillance (AS).
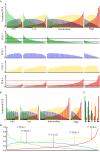
Patients and methods: Post-digital rectal examination urine-derived EV-RNA expression profiles (n = 535, multiple centres) were interrogated with a curated NanoString panel. A LASSO-based continuation ratio model was built to generate four prostate urine risk (PUR) signatures for predicting the probability of normal tissue (PUR-1), D'Amico low-risk (PUR-2), intermediate-risk (PUR-3), and high-risk (PUR-4) prostate cancer. This model was applied to a test cohort (n = 177) for diagnostic evaluation, and to an AS sub-cohort (n = 87) for prognostic evaluation.
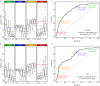
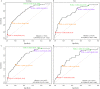
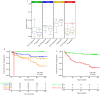
Results: Each PUR signature was significantly associated with its corresponding clinical category (P < 0.001). PUR-4 status predicted the presence of clinically significant intermediate- or high-risk disease (area under the curve = 0.77, 95% confidence interval [CI] 0.70-0.84). Application of PUR provided a net benefit over current clinical practice. In an AS sub-cohort (n = 87), groups defined by PUR status and proportion of PUR-4 had a significant association with time to progression (interquartile range hazard ratio [HR] 2.86, 95% CI 1.83-4.47; P < 0.001). PUR-4, when used continuously, dichotomized patient groups with differential progression rates of 10% and 60% 5 years after urine collection (HR 8.23, 95% CI 3.26-20.81; P < 0.001).
Conclusion: Urine-derived EV-RNA can provide diagnostic information on aggressive prostate cancer prior to biopsy, and prognostic information for men on AS. PUR represents a new and versatile biomarker that could result in substantial alterations to current treatment of patients with prostate cancer.
Keywords: #PCSM; #ProstateCancer; active surveillance; biomarker; cell free; liquid biopsy; urine.
© 2019 The Authors BJU International Published by John Wiley & Sons Ltd on behalf of BJU International.
Conflict of interest statement
A patent application has been filed by the authors for the present work. There are no other conflicts of interest to disclose.
Figures

References
-
- D'Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Cancer‐specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate‐specific antigen era. J Clin Oncol 2003; 21: 2163–72 - PubMed
-
- D'Amico AV, Whittington R, Bruce Malkowicz S et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. J Am Med Assoc 1998; 280: 969–74 - PubMed
-
- Gleason DFMG. Prediction of prognosis for prostatic staging, adenocarcinoma by combined histological grading and clinical. J Urol 1974; 111: 58–64 - PubMed
-
- Sanda MG, Cadeddu JA, Kirkby E et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part I: risk stratification, shared decision making, and care options. J Urol 2018; 199: 683–90 - PubMed
-
- Mottet N, Bellmunt J, Bolla M et al. EAU‐ESTRO‐SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 2017; 71: 618–29 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

