Role of B7H3/IL-33 Signaling in Pulmonary Fibrosis-induced Profibrogenic Alterations in Bone Marrow
- PMID: 31106564
- PMCID: PMC6794107
- DOI: 10.1164/rccm.201808-1560OC
Role of B7H3/IL-33 Signaling in Pulmonary Fibrosis-induced Profibrogenic Alterations in Bone Marrow
Abstract
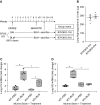
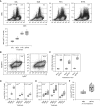
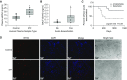
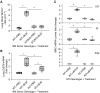
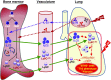
Rationale: The impact of lung insult on the bone marrow (BM) and subsequent disease is unknown.Objectives: To study alterations in the BM in response to lung injury/fibrosis and examine their impact on subsequent lung insult.Methods: BM cells from control or bleomycin-treated donor mice were transplanted into naive mice, which were subsequently evaluated for bleomycin-induced pulmonary fibrosis. In addition, the effect of prior bleomycin treatment on subsequent fibrosis was examined in wild-type and B7H3-knockout mice. Samples from patients with idiopathic pulmonary fibrosis were analyzed for potential clinical relevance of the findings.Measurements and Main Results: Recipient mice transplanted with BM from bleomycin-pretreated donors showed significant exacerbation of subsequent fibrosis with increased B7H3+ cell numbers and a T-helper cell type 2-skewed phenotype. Pretreatment with a minimally fibrogenic/nonfibrogenic dose of bleomycin also caused exacerbation, but not in B7H3-deficient mice. Exacerbation was not observed if the mice received naive BM cell transplant after the initial bleomycin pretreatment. Soluble B7H3 stimulated BM Ly6Chi monocytic cell expansion in vitro and caused similar expansion in the lung in vivo. Notably, soluble B7H3 was elevated in plasma of patients with idiopathic pulmonary fibrosis and in BAL fluid in those with acute exacerbation. Finally, ST2 deficiency diminished the bleomycin-induced B7H3 and IL-13 upregulation, suggesting a role for type 2 innate lymphoid cells.Conclusions: Pulmonary fibrosis caused significant alterations in BM with expansion and activation of monocytic cells, which enhanced fibrosis when transplanted to naive recipients with potential mediation by a novel role for B7H3 in the pathophysiology of pulmonary fibrosis in both mice and humans.
Keywords: bleomycin; bone marrow transplantation; group 2 innate lymphoid cells; idiopathic pulmonary fibrosis; monocytes.
Figures




Comment in
-
Remember Me? The Bone Marrow in Pulmonary Fibrosis.Am J Respir Crit Care Med. 2019 Oct 15;200(8):959-960. doi: 10.1164/rccm.201906-1101ED. Am J Respir Crit Care Med. 2019. PMID: 31206314 Free PMC article. No abstract available.
Similar articles
-
B7H3 expression and significance in idiopathic pulmonary fibrosis.J Pathol. 2022 Mar;256(3):310-320. doi: 10.1002/path.5838. Epub 2021 Dec 22. J Pathol. 2022. PMID: 34825713 Free PMC article.
-
B7H3-dependent myeloid-derived suppressor cell recruitment and activation in pulmonary fibrosis.Front Immunol. 2022 Aug 15;13:901349. doi: 10.3389/fimmu.2022.901349. eCollection 2022. Front Immunol. 2022. PMID: 36045668 Free PMC article.
-
An ST2-dependent role of bone marrow-derived group 2 innate lymphoid cells in pulmonary fibrosis.J Pathol. 2018 Aug;245(4):399-409. doi: 10.1002/path.5092. Epub 2018 Jun 5. J Pathol. 2018. PMID: 29722022
-
Characterization of lung stem cell niches in a mouse model of bleomycin-induced fibrosis.Stem Cell Res Ther. 2012 May 29;3(3):21. doi: 10.1186/scrt112. Stem Cell Res Ther. 2012. PMID: 22643035 Free PMC article.
-
Pulmonary fibrosis and type-17 immunity.Respir Investig. 2023 Sep;61(5):553-562. doi: 10.1016/j.resinv.2023.05.005. Epub 2023 Jun 23. Respir Investig. 2023. PMID: 37356133 Review.
Cited by
-
Antifibrotic effect of lung-resident progenitor cells with high aldehyde dehydrogenase activity.Stem Cell Res Ther. 2021 Aug 23;12(1):471. doi: 10.1186/s13287-021-02549-6. Stem Cell Res Ther. 2021. PMID: 34425896 Free PMC article.
-
B7H3 expression and significance in idiopathic pulmonary fibrosis.J Pathol. 2022 Mar;256(3):310-320. doi: 10.1002/path.5838. Epub 2021 Dec 22. J Pathol. 2022. PMID: 34825713 Free PMC article.
-
B7H3-dependent myeloid-derived suppressor cell recruitment and activation in pulmonary fibrosis.Front Immunol. 2022 Aug 15;13:901349. doi: 10.3389/fimmu.2022.901349. eCollection 2022. Front Immunol. 2022. PMID: 36045668 Free PMC article.
-
Mass cytometry identifies characteristic immune cell subsets in bronchoalveolar lavage fluid from interstitial lung diseases.Front Immunol. 2023 Mar 6;14:1145814. doi: 10.3389/fimmu.2023.1145814. eCollection 2023. Front Immunol. 2023. PMID: 36949950 Free PMC article.
-
The immune mechanisms of acute exacerbations of idiopathic pulmonary fibrosis.Front Immunol. 2024 Dec 16;15:1450688. doi: 10.3389/fimmu.2024.1450688. eCollection 2024. Front Immunol. 2024. PMID: 39737178 Free PMC article. Review.
References
-
- Satoh T, Nakagawa K, Sugihara F, Kuwahara R, Ashihara M, Yamane F, et al. Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature. 2017;541:96–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

