CEST MRI monitoring of tumor response to vascular disrupting therapy using high molecular weight dextrans
- PMID: 31106918
- PMCID: PMC7029807
- DOI: 10.1002/mrm.27818
CEST MRI monitoring of tumor response to vascular disrupting therapy using high molecular weight dextrans
Abstract
Purpose: Vascular disrupting therapy of cancer has become a promising approach not only to regress tumor growth directly but also to boost the delivery of chemotherapeutics in the tumor. An imaging approach to monitor the changes in tumor vascular permeability, therefore, has important applications for monitoring of vascular disrupting therapies.
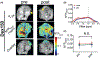
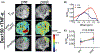
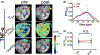
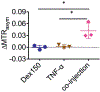
Methods: Mice bearing CT26 subcutaneous colon tumors were injected intravenously with 150 kD dextran (Dex150, diameter, d~ 20 nm, 375 mg/kg), tumor necrosis factor-alpha (TNF-α; 1 µg per mouse), or both (n = 3 in each group). The Z-spectra were acquired before and 2 h after the injection, and the chemical exchange saturation transfer (CEST) signals in the tumors as quantified by asymmetric magnetization transfer ratio (MTRasym ) at 1 ppm were compared.
Results: The results showed a significantly stronger CEST contrast enhancement at 1 ppm (∆MTRasym = 0.042 ± 0.002) in the TNF-α-treated tumors than those by Dex150 alone (∆MTRasym = 0.000 ± 0.005, P = 0.0229) or TNF-α alone (∆MTRasym = 0.002 ± 0.004, P = 0.0264), indicating that the TNF-α treatment strongly augmented the tumor uptake of 150 kD dextran. The MRI findings were verified by fluorescence imaging and immunofluorescence microscopy.
Conclusions: High molecular weight dextrans can be used as safe and sensitive CEST MRI contrast agents for monitoring tumor response to vascular disrupting therapy and, potentially, for developing dextran-based theranostic drug delivery systems.
Keywords: CEST; MRI; dextran; permeability; vascular disrupting therapy.
© 2019 International Society for Magnetic Resonance in Medicine.
Figures


Similar articles
-
Characterization of tumor vascular permeability using natural dextrans and CEST MRI.Magn Reson Med. 2018 Feb;79(2):1001-1009. doi: 10.1002/mrm.27014. Epub 2017 Nov 28. Magn Reson Med. 2018. PMID: 29193288 Free PMC article.
-
Dynamic contrast-enhanced CEST MRI using a low molecular weight dextran.NMR Biomed. 2022 Mar;35(3):e4649. doi: 10.1002/nbm.4649. Epub 2021 Nov 15. NMR Biomed. 2022. PMID: 34779550 Free PMC article.
-
CEST imaging of fast exchanging amine pools with corrections for competing effects at 9.4 T.NMR Biomed. 2017 Jul;30(7):10.1002/nbm.3715. doi: 10.1002/nbm.3715. Epub 2017 Mar 8. NMR Biomed. 2017. PMID: 28272785 Free PMC article.
-
Clinical applications of chemical exchange saturation transfer (CEST) MRI.J Magn Reson Imaging. 2018 Jan;47(1):11-27. doi: 10.1002/jmri.25838. Epub 2017 Aug 9. J Magn Reson Imaging. 2018. PMID: 28792646 Free PMC article. Review.
-
A review of optimization and quantification techniques for chemical exchange saturation transfer MRI toward sensitive in vivo imaging.Contrast Media Mol Imaging. 2015 May-Jun;10(3):163-178. doi: 10.1002/cmmi.1628. Epub 2015 Jan 12. Contrast Media Mol Imaging. 2015. PMID: 25641791 Free PMC article. Review.
Cited by
-
Repurposing Clinical Agents for Chemical Exchange Saturation Transfer Magnetic Resonance Imaging: Current Status and Future Perspectives.Pharmaceuticals (Basel). 2020 Dec 24;14(1):11. doi: 10.3390/ph14010011. Pharmaceuticals (Basel). 2020. PMID: 33374213 Free PMC article. Review.
-
Design Chemical Exchange Saturation Transfer Contrast Agents and Nanocarriers for Imaging Proton Exchange in Vivo.ACS Nano. 2024 Dec 17;18(50):33775-33791. doi: 10.1021/acsnano.4c05923. Epub 2024 Dec 6. ACS Nano. 2024. PMID: 39642940 Free PMC article. Review.
-
What do we know about dynamic glucose-enhanced (DGE) MRI and how close is it to the clinics? Horizon 2020 GLINT consortium report.MAGMA. 2022 Feb;35(1):87-104. doi: 10.1007/s10334-021-00994-1. Epub 2022 Jan 15. MAGMA. 2022. PMID: 35032288 Free PMC article. Review.
-
Contrasting Properties of Polymeric Nanocarriers for MRI-Guided Drug Delivery.Nanomaterials (Basel). 2023 Jul 25;13(15):2163. doi: 10.3390/nano13152163. Nanomaterials (Basel). 2023. PMID: 37570481 Free PMC article. Review.
-
Label-Free Assessment of Mannitol Accumulation Following Osmotic Blood-Brain Barrier Opening Using Chemical Exchange Saturation Transfer Magnetic Resonance Imaging.Pharmaceutics. 2022 Nov 20;14(11):2529. doi: 10.3390/pharmaceutics14112529. Pharmaceutics. 2022. PMID: 36432721 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

