The future of canine glaucoma therapy
- PMID: 31106969
- PMCID: PMC6744300
- DOI: 10.1111/vop.12678
The future of canine glaucoma therapy
Abstract
Canine glaucoma is a group of disorders that are generally associated with increased intraocular pressure (IOP) resulting in a characteristic optic neuropathy. Glaucoma is a leading cause of irreversible vision loss in dogs and may be either primary or secondary. Despite the growing spectrum of medical and surgical therapies, there is no cure, and many affected dogs go blind. Often eyes are enucleated because of painfully high, uncontrollable IOP. While progressive vision loss due to primary glaucoma is considered preventable in some humans, this is mostly not true for dogs. There is an urgent need for more effective, affordable treatment options. Because newly developed glaucoma medications are emerging at a very slow rate and may not be effective in dogs, work toward improving surgical options may be the most rewarding approach in the near term. This Viewpoint Article summarizes the discussions and recommended research strategies of both a Think Tank and a Consortium focused on the development of more effective therapies for canine glaucoma; both were organized and funded by the American College of Veterinary Ophthalmologists Vision for Animals Foundation (ACVO-VAF). The recommendations consist of (a) better understanding of disease mechanisms, (b) early glaucoma diagnosis and disease staging, (c) optimization of IOP-lowering medical treatment, (d) new surgical therapies to control IOP, and (e) novel treatment strategies, such as gene and stem cell therapies, neuroprotection, and neuroregeneration. In order to address these needs, increases in research funding specifically focused on canine glaucoma are necessary.
Keywords: aqueous humor; canine; glaucoma; intraocular pressure; optic nerve; surgery.
© 2019 The Authors. Veterinary Ophthalmology published by Wiley Periodicals, Inc. on behalf of American College of Veterinary Ophthalmologists.
Conflict of interest statement
The authors have the following potential conflicts of interest (in alphabetic order): Aerie Pharmaceutical (RLF and SEM: research funding, clinical trial grant), Allergan (SEM: research funding, clinical trial grant), Bausch and Lomb (RLF: research funding), Beaver‐Visitec (RLF: consultant), Cara Life (DWE: previous consultant), Elsevier (PEM: book royalties), Ivantis (CBT: research funding), Nicox (CBT: research funding), OSOD (PEM: consultant), PolyActiva (AMK: research funding), Santen (CBT: research funding), and Wolters Kluwer (SEM: book royalties). The opinions expressed in this article are the authors' own and do not necessarily reflect the view of the United States Food and Drug Administration (FDA) or the United States Government. Special thanks go to Ms. Jen Gazdacko (ACVO‐VAF) for her technical support.
Figures
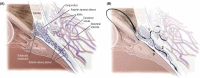
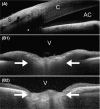





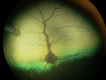
References
-
- Gelatt KN, MacKay EO. Prevalence of the breed‐related glaucomas in pure‐bred dogs in North America. Vet Ophthalmol. 2004;7:97‐111. - PubMed
-
- Gelatt KN, MacKay EO. Secondary glaucomas in the dog in North America. Vet Ophthalmol. 2004;7:245‐259. - PubMed
-
- Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta‐analysis. Ophthalmology. 2014;121:2081‐2090. - PubMed
-
- Newbold GM, Kelch WJ, Chen T, et al. Phacoemulsification outcomes in Boston terriers as compared to non‐Boston terriers: a retrospective study (2002–2015). Vet Ophthalmol. 2018;21:353‐361. - PubMed

