The role of macrophages in the resolution of inflammation
- PMID: 31107246
- PMCID: PMC6597225
- DOI: 10.1172/JCI124615
The role of macrophages in the resolution of inflammation
Abstract
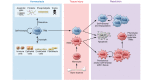
Macrophages are tissue-resident or infiltrated immune cells critical for innate immunity, normal tissue development, homeostasis, and repair of damaged tissue. Macrophage function is a sum of their ontogeny, the local environment in which they reside, and the type of injuries or pathogen to which they are exposed. In this Review, we discuss the role of macrophages in the restoration of tissue function after injury, highlighting important questions about how they respond to and modify the local microenvironment to restore homeostasis.
Conflict of interest statement
Figures