Clinical Consequences of Antibody Formation, Serum Concentrations, and HLA-Cw6 Status in Psoriasis Patients on Ustekinumab
- PMID: 31107404
- PMCID: PMC6752798
- DOI: 10.1097/FTD.0000000000000646
Clinical Consequences of Antibody Formation, Serum Concentrations, and HLA-Cw6 Status in Psoriasis Patients on Ustekinumab
Abstract
Background: Ustekinumab for the treatment of psoriasis is currently administered in a standard dosing regimen. However, some patients tend to benefit from alternative dosing regimens, a step toward personalized medicine.
Methods: To investigate the role of ustekinumab serum concentrations, anti-ustekinumab antibodies [AUA] and HLA-Cw6 status as tools for optimizing ustekinumab treatment, a multicenter prospective cohort study was conducted at an academic hospital with affiliated nonacademic hospitals in Belgium (cohort 1) and 2 academic hospitals in the Netherlands (cohort 2 and 3). Patients with plaque-type psoriasis were eligible if treated with ustekinumab for ≥16 weeks. Serum samples and Psoriasis Area and Severity Index scores were obtained at baseline, week 16, 28, 40, 52, and/or ≥64 of ustekinumab treatment.
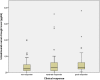
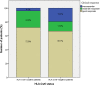
Results: A total of 137 patients with 229 observations for serum concentrations and AUA and 61 observations for HLA-Cw6 status were included. Presence of AUA (prevalence of 8.7%) was significantly associated with a diminished clinical response (P = 0.032). The median ustekinumab trough concentration was 0.3 mcg/mL (<0.02-3.80). No differences in serum concentrations were observed between moderate to good responders and nonresponders (P = 0.948). Serum trough concentrations were not affected by methotrexate comedication. Prevalence of HLA-Cw6 positivity was 41% with no statistically significant difference in clinical response between HLA-Cw6-positive and HLA-Cw6-negative patients (P = 0.164).
Conclusions: The presence of AUA was associated with treatment failure in this patient population; measurement of AUA may therefore be a candidate marker for personalized pharmacotherapy. The clinical utility of ustekinumab serum trough concentrations or HLA-Cw6 status determination remains less clear. Further exploration on the potential of measuring ustekinumab serum concentrations and other biomarkers in predicting therapy outcomes should be encouraged.
Figures

References
-
- Grine L, Dejager L, Libert C, et al. An inflammatory triangle in psoriasis: TNF, type I IFNs and IL-17. Cytokine Growth Factor Rev. 2015;26:25–33. - PubMed
-
- Benson JM, Sachs CW, Treacy G, et al. Therapeutic targeting of the IL-12/23 pathways: generation and characterization of ustekinumab. Nat Biotechnol. 2011;29:615–624. - PubMed
-
- Warren RB, Smith CH, Yiu ZZN, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol. 2015;135:2632–2640. - PubMed
-
- de la Brassinne M, Ghislain PD, Lambert JL, et al. Recommendations for managing a suboptimal response to biologics for moderate-to-severe psoriasis: a Belgian perspective. J Dermatolog Treat. 2016;27:128–133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

