Pharmacokinetics, safety, and efficacy of a single co-administered dose of diethylcarbamazine, albendazole and ivermectin in adults with and without Wuchereria bancrofti infection in Côte d'Ivoire
- PMID: 31107869
- PMCID: PMC6550417
- DOI: 10.1371/journal.pntd.0007325
Pharmacokinetics, safety, and efficacy of a single co-administered dose of diethylcarbamazine, albendazole and ivermectin in adults with and without Wuchereria bancrofti infection in Côte d'Ivoire
Abstract
Background: A single co-administered dose of ivermectin (IVM) plus diethylcarbamazine (DEC) plus albendazole (ALB), or triple-drug therapy, was recently found to be more effective for clearing microfilariae (Mf) than standard DEC plus ALB currently used for mass drug administration programs for lymphatic filariasis (LF) outside of sub-Saharan Africa. Triple-drug therapy has not been previously tested in LF-uninfected individuals from Africa. This study evaluated the pharmacokinetics (PK), safety, and efficacy of triple-drug therapy in people with and without Wuchereria bancrofti infection in West Africa.
Methods: In this open-label cohort study, treatment-naïve microfilaremic (>50 mf/mL, n = 32) and uninfected (circulating filarial antigen negative, n = 24) adults residing in Agboville district, Côte d'Ivoire, were treated with a single dose of IVM plus DEC plus ALB, and evaluated for adverse events (AEs) until 7 days post treatment. Drug levels were assessed by liquid chromatography and mass spectrometry. Persons responsible for assessing AEs were blinded to participants' infection status.
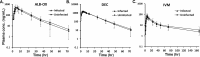
Findings: There was no difference in AUC0-inf or Cmax between LF-infected and uninfected participants (P>0.05 for all comparisons). All subjects experienced mild AEs; 28% and 25% of infected and uninfected participants experienced grade 2 AEs, respectively. There were no severe or serious adverse events. Only fever (16 of 32 versus 4 of 24, P<0.001) and scrotal pain/swelling in males (6 of 20 versus 0 of 12, P = 0.025) were more frequent in infected than uninfected participants. All LF positive participants were amicrofilaremic at 7 days post-treatment and 27 of 31 (87%) remained amicrofilaremic 12 months after treatment.
Conclusions: Moderate to heavy W. bancrofti infection did not affect PK parameters for IVM, DEC or ALB following a single co-administered dose of these drugs compared to uninfected individuals. The drugs were well tolerated. This study confirmed the efficacy of the triple-drug therapy for clearing W. bancrofti Mf and has added important information to support the use of this regimen in LF elimination programs in areas of Africa without co-endemic onchocerciasis or loiasis.
Trial registration: ClinicalTrials.gov NCT02845713.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- WHO. Global programme to eliminate lymphatic filariasis: progress report. Wkly Epidemiol Rec. 2017;92(40):594–607. - PubMed
-
- Ottesen EA. The global programme to eliminate lymphatic filariasis. Tropical medicine & international health: TM & IH. 2000;5(9):591–4. Epub 2000/10/24. tmi620 [pii]. . - PubMed
-
- WHO. The Global Programme to Eliminate Lymphatic Filariasis: Progress Report 2000–2009 and Strategic Plan 2010–2020. Geneva: 2011.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

