The evolution and multi-molecular properties of NF1 cutaneous neurofibromas originating from C-fiber sensory endings and terminal Schwann cells at normal sites of sensory terminations in the skin
- PMID: 31107888
- PMCID: PMC6527217
- DOI: 10.1371/journal.pone.0216527
The evolution and multi-molecular properties of NF1 cutaneous neurofibromas originating from C-fiber sensory endings and terminal Schwann cells at normal sites of sensory terminations in the skin
Abstract
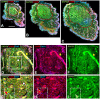
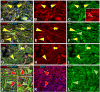
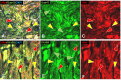
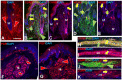
In addition to large plexiform neurofibromas (pNF), NF1 patients are frequently disfigured by cutaneous neurofibromas (cNF) and are often afflicted with chronic pain and itch even from seemingly normal skin areas. Both pNFs and cNF consist primarily of benign hyperproliferating nonmyelinating Schwann cells (nSC). While pNF clearly arise within deep nerves and plexuses, the role of cutaneous innervation in the origin of cNF and in chronic itch and pain is unknown. First, we conducted a comprehensive, multi-molecular, immunofluorescence (IF) analyses on 3mm punch biopsies from three separate locations in normal appearing, cNF-free skin in 19 NF1 patients and skin of 16 normal subjects. At least one biopsy in 17 NF1 patients had previously undescribed micro-lesions consisting of a small, dense cluster of nonpeptidergic C-fiber endings and the affiliated nSC consistently adjoining adnexal structures-dermal papillae, hair follicles, sweat glands, sweat ducts, and arterioles-where C-fiber endings normally terminate. Similar micro-lesions were detected in hind paw skin of mice with conditionally-induced SC Nf1-/- mutations. Hypothesizing that these microlesions were pre-cNF origins of cNF, we subsequently analyzed numerous overt, small cNF (s-cNF, 3-6 mm) and discovered that each had an adnexal structure at the epicenter of vastly increased nonpeptidergic C-fiber terminals, accompanied by excessive nSC. The IF and functional genomics assays indicated that neurturin (NTRN) and artemin (ARTN) signaling through cRET kinase and GFRα2 and GFRα3 co-receptors on the aberrant C-fiber endings and nSC may mutually promote the onset of pre-cNF and their evolution to s-cNF. Moreover, TrpA1 and TrpV1 receptors may, respectively, mediate symptoms of chronic itch and pain. These newly discovered molecular characteristics might be targeted to suppress the development of cNF and to treat chronic itch and pain symptoms in NF1 patients.
Conflict of interest statement
FLR and PJA are the co-founders and co-owners of INTiDYN, which is a for-profit contract and private equity research and consulting organization. GH and MD are research employees of INTiDYN. INTiDYN has no other commercial affiliations but does conduct contract research for pharmaceutical companies and academic collaborators unrelated to, and having no conflict of interest in this research. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
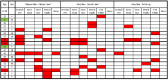

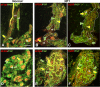

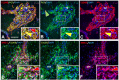
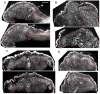





References
-
- Riccardi VM. Neurofibromatosis: Phenotype, Natural History and Pathogenesis. Baltimore: Johns Hopkins University Press; 1992. 1–498 p.
-
- Riccardi VM. Translational Genetics and Genomics: The Fundamental Nature of NF1 Neurofibromas. JTranslGenetGenom. 2017;1(1):1–12. Epub 2/123/2017.
-
- Tucker T, Riccardi VM, Brown C, Fee J, Sutcliffe M, Vielkind J, et al. S100B and neurofibromin immunostaining and X-inactivation patterns of laser microdissected cells indicate a multicellular origin of some NF1-associated neurofibromas. Journal of Neuroscience Research. 2011;89:1451–60. 10.1002/jnr.22654 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

