Carvedilol inhibits EGF-mediated JB6 P+ colony formation through a mechanism independent of adrenoceptors
- PMID: 31107911
- PMCID: PMC6527222
- DOI: 10.1371/journal.pone.0217038
Carvedilol inhibits EGF-mediated JB6 P+ colony formation through a mechanism independent of adrenoceptors
Abstract
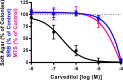
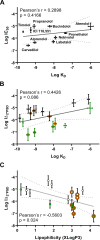
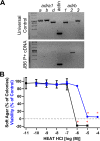
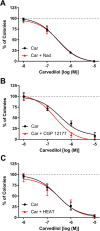
Carvedilol is reported to prevent cancers in humans and animal models. However, a molecular mechanism has yet to be established, and the extent to which other β-blockers are chemopreventive remains relatively unknown. A comparative pharmacological approach was utilized with the expectation that a mechanism of action could be devised. JB6 Cl 41-5a (JB6 P+) murine epidermal cells were used to elucidate the chemopreventative properties of β-blockers, as JB6 P+ cells recapitulate in vivo tumor promotion and chemoprevention. The initial hypothesis was that β-blockers that are GRK/β-arrestin biased agonists, like carvedilol, are chemopreventive. Sixteen β-blockers of different classes, isoproterenol, and HEAT HCl were individually co-administered with epidermal growth factor (EGF) to JB6 P+ cells to examine the chemopreventative properties of each ligand. Cytotoxicity was examined to ensure that the anti-transformation effects of each ligand were not due to cellular growth inhibition. Many of the examined β-blockers suppressed EGF-induced JB6 P+ cell transformation in a non-cytotoxic and concentration-dependent manner. However, the IC50 values are high for the most potent inhibitors (243, 326, and 431 nM for carvedilol, labetalol, and alprenolol, respectively) and there is no correlation between pharmacological properties and inhibition of transformation. Therefore, the role of α1- and β2-adrenergic receptors (AR) was examined by standard competition assays and shRNA targeting β2-ARs, the only β-AR expressed in JB6 P+ cells. The results reveal that pharmacological inhibition of α1- and β2-ARs and genetic knockdown of β2-ARs did not abrogate carvedilol-mediated inhibition of EGF-induced JB6 P+ cell transformation. Furthermore, topical administration of carvedilol protected mice from UV-induced skin damage, while genetic ablation of β2-ARs increased carvedilol-mediated effects. Therefore, the prevailing hypothesis that the chemopreventive property of carvedilol is mediated through β-ARs is not supported by this data.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
The β-Blocker Carvedilol Prevented Ultraviolet-Mediated Damage of Murine Epidermal Cells and 3D Human Reconstructed Skin.Int J Mol Sci. 2020 Jan 25;21(3):798. doi: 10.3390/ijms21030798. Int J Mol Sci. 2020. PMID: 31991834 Free PMC article.
-
Prevention of skin carcinogenesis by the β-blocker carvedilol.Cancer Prev Res (Phila). 2015 Jan;8(1):27-36. doi: 10.1158/1940-6207.CAPR-14-0193. Epub 2014 Nov 3. Cancer Prev Res (Phila). 2015. PMID: 25367979 Free PMC article.
-
Phosphoproteome profiling provides insight into the mechanism of action for carvedilol-mediated cancer prevention.Mol Carcinog. 2018 Aug;57(8):997-1007. doi: 10.1002/mc.22820. Epub 2018 Apr 24. Mol Carcinog. 2018. PMID: 29626349
-
Roles of β-adrenoceptor Subtypes and Therapeutics in Human Cardiovascular Disease: Heart Failure, Tachyarrhythmias and Other Cardiovascular Disorders.Handb Exp Pharmacol. 2024;285:247-295. doi: 10.1007/164_2024_720. Handb Exp Pharmacol. 2024. PMID: 38844580 Review.
-
Carvedilol: a new candidate for reversal of MDR1/P-glycoprotein-mediated multidrug resistance.Anticancer Drugs. 2004 Apr;15(4):303-9. doi: 10.1097/00001813-200404000-00001. Anticancer Drugs. 2004. PMID: 15057133 Review.
Cited by
-
The β-Blocker Carvedilol Prevented Ultraviolet-Mediated Damage of Murine Epidermal Cells and 3D Human Reconstructed Skin.Int J Mol Sci. 2020 Jan 25;21(3):798. doi: 10.3390/ijms21030798. Int J Mol Sci. 2020. PMID: 31991834 Free PMC article.
-
Effects of carvedilol on human prostate tissue contractility and stromal cell growth pointing to potential clinical implications.Pharmacol Rep. 2024 Aug;76(4):807-822. doi: 10.1007/s43440-024-00605-5. Epub 2024 Jun 11. Pharmacol Rep. 2024. PMID: 38858312 Free PMC article.
-
The β-Blocker Carvedilol Prevents Benzo(a)pyrene-Induced Lung Toxicity, Inflammation and Carcinogenesis.Cancers (Basel). 2023 Jan 18;15(3):583. doi: 10.3390/cancers15030583. Cancers (Basel). 2023. PMID: 36765542 Free PMC article.
-
Investigating Carvedilol's Repurposing for the Treatment of Non-Small Cell Lung Cancer via Aldehyde Dehydrogenase Activity Modulation in the Presence of β-Adrenergic Agonists.Curr Issues Mol Biol. 2023 Sep 29;45(10):7996-8012. doi: 10.3390/cimb45100505. Curr Issues Mol Biol. 2023. PMID: 37886948 Free PMC article.
-
Transfersome Encapsulated with the R-carvedilol Enantiomer for Skin Cancer Chemoprevention.Nanomaterials (Basel). 2023 Mar 3;13(5):929. doi: 10.3390/nano13050929. Nanomaterials (Basel). 2023. PMID: 36903807 Free PMC article.
References
-
- Cakir Y, Plummer HK III, Tithof PK, Schuller HM. Beta-adrenergic and arachidonic acid-mediated growth regulation of human breast cancer cell lines. IntJOncol. 2002;21(1):153–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

