Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease
- PMID: 31107966
- PMCID: PMC6527426
- DOI: 10.1002/14651858.CD001287.pub6
Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease
Abstract
Background: Individuals with chronic bronchitis or chronic obstructive pulmonary disease (COPD) may suffer recurrent exacerbations with an increase in volume or purulence of sputum, or both. Personal and healthcare costs associated with exacerbations indicate that therapies that reduce the occurrence of exacerbations are likely to be useful. Mucolytics are oral medicines that are believed to increase expectoration of sputum by reducing its viscosity, thus making it easier to cough it up. Improved expectoration of sputum may lead to a reduction in exacerbations of COPD.
Objectives: Primary objective• To determine whether treatment with mucolytics reduces exacerbations and/or days of disability in patients with chronic bronchitis or COPDSecondary objectives• To assess whether mucolytics lead to improvement in lung function or quality of life• To determine frequency of adverse effects associated with use of mucolytics SEARCH METHODS: We searched the Cochrane Airways Group Specialised Register and reference lists of articles on 12 separate occasions, most recently on 23 April 2019.
Selection criteria: We included randomised studies that compared oral mucolytic therapy versus placebo for at least two months in adults with chronic bronchitis or COPD. We excluded studies of people with asthma and cystic fibrosis.
Data collection and analysis: This review analysed summary data only, most derived from published studies. For earlier versions, one review author extracted data, which were rechecked in subsequent updates. In later versions, review authors double-checked extracted data and then entered data into RevMan 5.3 for analysis.
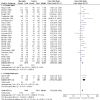
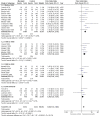
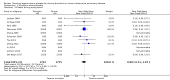
Main results: We added four studies for the 2019 update. The review now includes 38 trials, recruiting a total of 10,377 participants. Studies lasted between two months and three years and investigated a range of mucolytics, including N-acetylcysteine, carbocysteine, erdosteine, and ambroxol, given at least once daily. Many studies did not clearly describe allocation concealment, and we had concerns about blinding and high levels of attrition in some studies. The primary outcomes were exacerbations and number of days of disability.Results of 28 studies including 6723 participants show that receiving mucolytics may be more likely to be exacerbation-free during the study period compared to those given placebo (Peto odds ratio (OR) 1.73, 95% confidence interval (CI) 1.56 to 1.91; moderate-certainty evidence). However, more recent studies show less benefit of treatment than was reported in earlier studies in this review. The overall number needed to treat with mucolytics for an average of nine months to keep an additional participant free from exacerbations was eight (NNTB 8, 95% CI 7 to 10). High heterogeneity was noted for this outcome (I² = 62%), so results need to be interpreted with caution. The type or dose of mucolytic did not seem to alter the effect size, nor did the severity of COPD, including exacerbation history. Longer studies showed smaller effects of mucolytics than were reported in shorter studies.Mucolytic use was associated with a reduction of 0.43 days of disability per participant per month compared with use of placebo (95% CI -0.56 to -0.30; studies = 9; I² = 61%; moderate-certainty evidence). With mucolytics, the number of people with one or more hospitalisations was reduced, but study results were not consistent (Peto OR 0.68, 95% CI 0.52 to 0.89; participants = 1788; studies = 4; I² = 58%; moderate-certainty evidence). Investigators reported improved quality of life with mucolytics (mean difference (MD) -1.37, 95% CI -2.85 to 0.11; participants = 2721; studies = 7; I² = 64%; moderate-certainty evidence). However, the mean difference did not reach the minimal clinically important difference of -4 units, and the confidence interval includes no difference. Mucolytic treatment was associated with a possible reduction in adverse events (OR 0.84, 95% CI 0.74 to 0.94; participants = 7264; studies = 24; I² = 46%; moderate-certainty evidence), but the pooled effect includes no difference if a random-effects model is used. Several studies that could not be included in the meta-analysis reported high numbers of adverse events, up to a mean of five events per person during follow-up. There was no clear difference between mucolytics and placebo for mortality, but the confidence interval is too wide to confirm that treatment has no effect on mortality (Peto OR 0.98, 95% CI 0.51 to 1.87; participants = 3527; studies = 11; I² = 0%; moderate-certainty evidence).
Authors' conclusions: In participants with chronic bronchitis or COPD, we are moderately confident that treatment with mucolytics leads to a small reduction in the likelihood of having an acute exacerbation, in days of disability per month and possibly hospitalisations, but is not associated with an increase in adverse events. There appears to be limited impact on lung function or health-related quality of life. Results are too imprecise to be certain whether or not there is an effect on mortality. Our confidence in the results is reduced by high levels of heterogeneity in many of the outcomes and the fact that effects on exacerbations shown in early trials were larger than those reported by more recent studies. This may be a result of greater risk of selection or publication bias in earlier trials, thus benefits of treatment may not be as great as was suggested by previous evidence.
Conflict of interest statement
PP: none known. I am an editor with Cochrane Airways.
KS: none known.
RF: none known. I am Joint Co‐ordinating Editor of Cochrane Airways, employed by an NIHR grant, and a qualified general practitioner.
Figures























Update of
-
Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2015 Jul 29;(7):CD001287. doi: 10.1002/14651858.CD001287.pub5. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2019 May 20;5:CD001287. doi: 10.1002/14651858.CD001287.pub6. PMID: 26222376 Updated.
References
References to studies included in this review
Allegra 1996 {published and unpublished data}
-
- Allegra L, Cordaro CI, Grassi C. Prevention of acute exacerbations of chronic obstructive bronchitis with carbocysteine lysine salt monohydrate: a multicenter, double‐blind, placebo‐controlled trial. Respiration 1996;63:174‐80. - PubMed
Babolini 1980 {published data only}
-
- Multicenter Study Group. Long‐term oral acetylcysteine in chronic bronchitis. A double‐blind controlled study. European Journal of Respiratory Disease 1980;61(111 Suppl):93‐108. - PubMed
Bachh 2007 {published data only}
-
- Bachh AA, Shah NN, Bhargava R, Ahmad Z, Pandey DK, Dar KA. Effect of oral N‐acetylcysteine in COPD ‐ a randomised controlled trial. JK‐Practitioner 2007;14(1):12‐6.
Boman 1983 {published data only}
-
- Boman G, Backer U, Larsson S, Melander B, Wahlander L. Oral acetylcysteine reduces exacerbation rate in chronic bronchitis; report of a trial organized by the Swedish Society for Pulmonary Diseases. European Journal of Respiratory Disease 1983;64:405‐15. - PubMed
Bontognali 1991 {published data only}
-
- Bontognali E. Clinical effectiveness and tolerance of cithiolone in the prophylaxis of acute infective exacerbations in patients suffering from chronic bronchitis. Acta Therapeutica 1991;17:155‐62.
Borgia 1981 {published data only}
-
- Borgia M, Sepe N, Ori‐Belometti M, Borgia R. Comparison between acetylcysteine and placebo in the long term treatment of chronic bronchitis [Confronto tra acetilcisteina e placebo nel trattamento a lungo termine della bronchite cronica]. Gazzetta Medica Italiana 1981;140:467‐72.
Castiglioni 1986 {published data only}
-
- Castiglioni CL, Gramolini C. Effect of long‐term treatment with sobrerol on the exacerbations of chronic bronchitis. Respiration 1986;50:202‐17. - PubMed
Cegla 1988 {published data only}
-
- Cegla UH. Long‐term treatment of chronic bronchitis for two years with ambroxol (Mucosolvan) retard capsules. Results of a double blind trial including 180 patients [Langzeittherapie uber 2 Jahre mit Ambroxol (Mucosolvan) Retardkapseln bei Patienten mit chronischer Bronchitis. Ergebnisse einer Doppelblindstudie an 180 Patienten]. Praxis Klinik der Pneumologie 1988;42:715‐21. - PubMed
Cremonini 1986 {published data only}
-
- Cremonini C, Spada E, Cellini F, Cioni R, Giovannini M, Perri G, et al. I farmaci attivi sul muco nel trattamento di fondo della bronchite cronica [Pharmacotherapy which acts on mucus in long‐term treatment of chronic bronchitis]. La Clinica Terapeutica 1986;116:121‐9. - PubMed
Dal Negro 2017 {published data only}
-
- Calverley PMA, Page C, Dal Negro RW, Fontana G, Iversen M, Cicero AF, et al. Effect of erdosteine in moderately severe COPD patients. European Respiratory Journal. 2018; Vol. 52:PA776.
-
- Calverley PMA, Page C, Dal Negro RW, Fontana G, Iversen M, Cicero AF, et al. Effect of erdosteine on the rate of COPD exacerbations in spirometrically defined Gold II patients. Respirology. 2017; Vol. 2:49.
-
- Dal Negro RW, Iversen M, Calverly PM. Efficacy and safety of erdostein in COPD: results of a 12‐month prospective, multinational study. European Respiratory Journal 2015;46:PA1495.
-
- Dal Negro RW, Wedzicha JA, Iversen MI, Fontana G, Page C, Cicero AF, et al. Effect of erdosteine on the rate and duration of COPD exacerbations: the RESTORE study (Reducing Exacerbations and Symptoms by Treatment with ORal Erdosteine). European Respiratory Journal. 2017; Vol. 50:PA675. - PMC - PubMed
De Backer 2013 {published data only}
-
- Backer J, Vos W, Holsbeke C, Vinchurkar S, Claes R, Parizel P, et al. Double blind, randomized, two‐way crossover, pilot study to assess the effect of high dose N‐acetylcysteine on airway geometry, inflammation and oxidative stress in COPD patients using functional respiratory imaging. American Journal of Respiratory and Critical Care Medicine. 2013; Vol. 187, issue A2447.
Decramer 2005 {published data only}
-
- Decramer M, Rutten‐van Molken M, Dekhuijzen PN, Troosters T, Herwaarden C, Pellegrino R, et al. Effects of N‐acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost‐Utility Study, BRONCUS): a randomised placebo‐controlled trial. Lancet 2005;365(9470):1552‐60. - PubMed
Ekberg‐Jansson 1999 {published data only}
-
- Ekberg‐Jansson A, Larson M, MacNee W, Tunek A, Wahlgren L, Wouters EFM, et al. N isobutyrylcysteine, a donor of systemic thiols, does not reduce the exacerbation rate in chronic bronchitis. European Respiratory Journal 1999;13(4):829‐34. - PubMed
Fukuchi 2016 {published data only}
Grassi 1976 {published data only}
-
- Grassi C, Morandini GC. A controlled trial of intermittent oral acetylcysteine in the long‐term treatment of chronic bronchitis. European Journal of Clinical Pharmacology 1976;9:393‐6. - PubMed
Grassi 1994 {published data only}
-
- Grassi C, Casali L, Ciaccia A, et al. [Terapia intervallare con l'associazione carbocisteina‐sobrerolo nella profilassi delle riacutizzazioni della bronchite cronica]. Italian Journal of Chest Disease 1994;48:17‐26.
Grillage 1985 {published data only}
-
- Grillage M, Barnard‐Jones K. Long‐term oral carbocisteine therapy in patients with chronic bronchitis. A double blind trial with placebo control. British Journal of Clinical Practice 1985;39(10):395‐8. - PubMed
Hansen 1994 {published data only}
-
- Hansen NC, Skriver A, Brorsen‐Riis L, Balsløv S, Evald T, Maltbaek N, et al. Orally administered N‐acetylcysteine may improve general well‐being in patients with mild chronic bronchitis. Respiratory Medicine 1994;88:531‐5. - PubMed
Jackson 1984 {published data only}
-
- Jackson IM, Barnes J, Cooksey P. Efficacy and tolerability of oral acetylcysteine (fabrol) in chronic bronchitis: a double‐blind placebo controlled trial. Journal of International Medical Research 1984;12:198‐206. - PubMed
Johnson 2016 {published data only}
-
- Johnson K, McEvoy CE, Naqvi S, Wendt C, Reilkoff RA, Kunisaki KM, et al. High‐dose oral N‐acetylcysteine fails to improve respiratory health status in patients with chronic obstructive pulmonary disease and chronic bronchitis: a randomized, placebo‐controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:799‐807. - PMC - PubMed
Malerba 2004 {published data only}
-
- Malerba M, Ponticello A, Radaeli A, Bensi G, Grassi V. Effect of twelve‐months therapy with oral ambroxol in preventing exacerbations in patients with COPD. Double‐blind, randomized, multi‐center, placebo‐controlled study (the AMETHIST trial). Pulmonary Pharmacology and Therapeutics 2004;17(1):27‐34. - PubMed
McGavin 1985 {published data only}
Meister 1986 {unpublished data only}
-
- Meister R. Long‐term therapy with acetylcysteine retard tablets in patients with chronic bronchitis: a double‐blind, placebo controlled study. Unpublished source 1986.
Meister 1999 {published data only}
-
- Beeh K‐M, Beier J, Candler H, Wittig T. Effect of ELOM‐080 on exacerbations and symptoms in COPD patients with a chronic bronchitis phenotype ‐ a post‐hoc analysis of a randomized, double‐blind, placebo‐controlled clinical trial. International Journal of Chronic Obstructive Pulmonary Disease 2016;11(1):2877‐84. - PMC - PubMed
-
- Meister R, Wittig T, Beuscher N, Mey C. Efficacy and tolerability of myrtol standardized in long‐term treatment of chronic bronchitis. A double‐blind, placebo‐controlled study. Study Group Investigators. Arzneimittelforschung 1999;49(4):351‐8. - PubMed
Moretti 2004 {published data only}
-
- Moretti M, Bottrighi P, Dallari R, Porto R, Dolcetti A, Grandi P, et al. The effect of long‐term treatment with erdosteine on chronic obstructive pulmonary disease: the EQUALIFE study. Drugs Under Experimental and Clinical Research 2004;30(4):143‐52. - PubMed
Nowak 1999 {published data only}
-
- Nowak D, Carati L, Pirozynski M. Long‐term administration of N‐acetylcysteine reduces the number of acute exacerbation episodes in subjects with chronic obstructive pulmonary disease:report of the BREATHE study. European Respiratory Journal 1999;14 Suppl:381S‐2S.
Olivieri 1987 {published data only}
-
- Olivieri D, Zavattini G, Tomasini G, Daniotti S, Bonsignore G, Ferrara G, et al. Ambroxol for the prevention of chronic bronchitis exacerbations: long‐term multicenter trial. Respiration 1987;51 Suppl 1:42‐51. - PubMed
Parr 1987 {published data only}
-
- Parr GD, Huitson A. Oral fabrol (oral n‐acetyl‐cysteine) in chronic bronchitis. British Journal of Diseases of the Chest 1987;81(4):341‐8. - PubMed
Pela 1999 {published data only}
-
- Pela R, Calcagni AM, Subiaco S, Isidori P, Tubaldi A, Sanguinetti CM. N‐acetylcysteine reduces the exacerbation rate in patients with moderate to severe COPD. Respiration 1999;66(6):495‐500. - PubMed
Petty 1990 {published data only}
Rasmussen 1988 {published data only}
-
- Rasmussen JB, Glennow C. Reduction in days of illness after long‐term treatment with N‐acetylcysteine controlled‐release tablets in patients with chronic bronchitis. European Respiratory Journal 1988;1:351‐5. - PubMed
Roy 2014 {published data only}
-
- Roy P, Haran A, Srinivas BN. Role of n‐acetylcysteine as an adjuvant to mainstay bronchodilator therapy in chronic obstructive pulmonary disease. Indian Journal of Pharmacology Conference Publication 2013;45:S107.
-
- Roy P, Haran A, Srinivas BN, Mjumdar P. Evaluation of effect of N‐acetylcysteine as an adjuvant to mainstay bronchodilator therapy in mild to moderate cases of chronic obstructive pulmonary disease. International Journal of Medical and Applied Sciences 2014;3(1):95‐105.
Schermer 2009 {published data only}
-
- Chavannes N, Schermer T, Wouters EFM, Folgering H. Demographic and clinical determinants of response to N‐acetylcysteine versus fluticasone in mild to moderate COPD in primary care: the COOPT study. European Respiratory Journal 2005;26 (S49):1356.
-
- Schermer T, Chavannes N, Dekhuijzen R, Wouters E, Muris J, Akkermans R, et al. Fluticasone and N‐acetylcysteine in primary care patients with COPD or chronic bronchitis. Respiratory Medicine 2009;103:542‐51. - PubMed
Tse 2013 {published data only}
-
- Tse HN, Raiteri L, Wong KY, Ng LY, Yee Ks, Tseng CZS. Benefits of high‐dose N‐acetylcysteine to exacerbation‐prone patients with COPD. Chest 2014;146(3):611‐23. - PubMed
-
- Tse HN, Raiteri L, Wong KY, Yee KS, Ng LY, Wai KY, et al. High‐dose N‐acetylcysteine in stable COPD: the 1‐year, double‐blind, randomized, placebo‐controlled HIACE study. Chest 2013;144:106‐18. - PubMed
-
- Tse HN, Wong KY, Yee KS, Ng LY. The effect of high dose N‐acetylcysteine (1200mg daily) on airway function and airway trapping in COPD patients: a double blinded randomized placebo controlled trial. European Respiratory Journal 2012;40 Suppl 56:P2105.
-
- Tse HN, Wong KY, Yee KS, Ng LY, Wai KY, Loo CK, et al. The effect of high dose N‐acetylcysteine (600 mg twice daily) in patients with stable chronic obstructive pulmonary disease ‐ a one‐year, double blind, randomized, placebo‐controlled trial. American Journal of Respiratory and Critical Care Medicine 2013;187:A2448.
Worth 2009 {published data only}
-
- Worth H, Schacher C, Dethlefsen U. Effects of concomitant therapy with Cineole (Eucalyptole) on exacerbation rate in COPD: a placebo‐controlled double‐blind trial. European Respiratory Society 20th Annual Congress; 2010 Sep 18‐22; Barcelona. 2010; Vol. P538.
Xu 2014 {published data only}
-
- Xu X‐G, Jiang Z‐Y, Du M‐J, Yang Y‐Q, Jiang Y‐C. Evaluation on effectiveness of salmeterol/fluticasone propionate combined with N‐acetylcysteine in treatment of chronic obstructive pulmonary disease. [Chinese]. Journal of Jilin University Medicine Edition 2014;40(40):870‐4.
Zheng 2008 {published data only}
-
- Zheng JP, Kang J, Huang SG, Chen P, Yao WZ, Yang L, et al. Effect of carbocisteine on acute exacerbations of chronic obstructive pulmonary disease (PEACE study): a randomised placebo‐controlled trial. Lancet 2008;371(9629):2013‐8. - PubMed
Zheng 2014 {published data only}
-
- Papi A, Brusselle G, Sergio F, Pannacci M, Zheng J, Criner G. Blood eosinophils and smoking history affect outcome with high dose N‐acetylcysteine treatment in COPD. European Respiratory Journal. 2017; Vol. 50:PA1066.
-
- Papi A, Zheng J, Criner GJ, Fabbri LM, Calverley PMA. Impact of smoking status and concomitant medications on the effect of high‐dose N‐acetylcysteine on chronic obstructive pulmonary disease exacerbations: a post‐hoc analysis of the PANTHEON study. Respiratory Medicine 2019;147:37‐43. - PubMed
-
- Sergio F, Papi A, Criner G, Brusselle G, Calverley P, Fabbri L, et al. Long term treatment with n‐acetylcysteine reduces moderate‐severe exacerbations of chronic obstructive pulmonary disease. Respirology 2016;21:186.
-
- Zheng JP, Wen FQ, Bai CX, Wan HY, Kang J, Chen P, et al. High‐dose N‐acetylcysteine in the prevention of COPD exacerbations: rationale and design of the PANTHEON study. COPD: Journal of Chronic Obstructive Pulmonary Disease 2013;10:164‐71. - PubMed
-
- Zheng JP, Wen FQ, Bai CX, Wan HY, Kang J, Chen P, et al. High‐dose N‐acetylcysteine in the prevention of COPD exacerbations: results of the PANTHEON study. European Respiratory Society 23rd Annual Congress; 2013 Sep 7‐11; Barcelona. 2013.
References to studies excluded from this review
Baglioni 2001 {published data only}
-
- Baglioni S, Tazza R, Rossi S, Eslami A, Ferranti P, Dottorini M. Effects of N‐acetylcysteine treatment during long term oxygen therapy (LTOT) in COPD patients. An open parallel‐group study. European Respiratory Journal 2001;18(33 Suppl):58S.
Cattaneo 2001 {published data only}
-
- Cattaneo C. Neltenexine tablets in smoking and non‐smoking patients with COPD. A double‐blind, randomised, controlled study versus placebo. Minerva Medica 2001;92(4):277‐84. - PubMed
Christensen 1971 {published data only}
-
- Andreasen T. Mucolytic treatment of chronic bronchitis during two winter periods. Scandinavian Journal of Respiratory Disease 1974;90:69‐70. - PubMed
-
- Christensen SB, Kjer J, Ryskjaer S, Arseth‐Hansen P, Christensen F. Mucolytic treatment of chronic bronchitis during two winter periods. Scandinavian Journal of Respiratory Disease 1971;52(1):48‐57. - PubMed
Edwards 1976 {published data only}
-
- Edwards GF, Steel AE, Scott JK, Jordan JW. S‐carboxymethylcysteine in the fluidification of sputum and treatment of chronic airway obstruction. Chest 1976;70(4):506‐13. - PubMed
Habich 1994 {published data only}
-
- Habich G, Repges R. Chronic obstructive lung diseases: the efficacy of cineole [Cineol als medikation sinnvoll und bewart]. Therapiewoche 1994;44(6):356‐65.
Kasielski 2001 {published data only}
-
- Kasielski M, Nowak D. Long‐term administration of N‐acetylcysteine decreases hydrogen peroxide exhalation in subjects with chronic obstructive pulmonary disease. Respiratory Medicine 2001;95(6):448‐56. - PubMed
Lukas 2005 {published data only}
-
- Lukas R, Scharling B, Schultze‐Werninghaus G, Gillisen A. Administration of N‐acetylcysteine and vitamin C to augment antioxidant properties in patients with chronic bronchitis. Deutsche Medizinishe Wochenscrift 2005;130:563‐7. - PubMed
Maesen 1980 {published data only}
-
- Maesen FP, Brombacher PJ. Treatment of chronic bronchitis with oral acetylcysteine, a double‐blind study. European Journal of Respiratory Disease 1980;61:110.
Michnar 1996 {published data only}
-
- Michnar M, Milanowski J. Assessment of efficacy and tolerability of the oral treatment of ambrosol in patients with chronic bronchitis [Ocena kliniczna skutecznosci i tolerancji doustnego leczenia ambrosolem u chorych na przewlekle zapalenie oskrzeli]. Pneumonologia i Alergologia Polska 1996;64(Suppl 1):90‐6. - PubMed
Moretti 2011 {unpublished data only}
-
- Moretti M, Ballabio M. Effect of erdosteine on airflow obstruction and symptom recovery in severe COPD exacerbations [Abstract]. European Respiratory Society 21st Annual Congress; 2011 Sep 24‐28; Amsterdam. 2011; Vol. 38 (55):76s [P536].
Moretti 2014 {published data only}
-
- Moretti M. Erdosteine oral treatment of hospitalised COPD exacerbation prolongs time to first re‐exacerbation [Abstract]. European Respiratory Journal 2014;44:P293.
Pirabbasi 2016 {published data only}
-
- Pirabbasi E, Shahar S, Manaf ZA, Rajab NF, Manap RA. Efficacy of ascorbic acid (vitamin C) and/N‐acetylcysteine (NAC) supplementation on nutritional and antioxidant status of male chronic obstructive pulmonary disease (COPD) patients. Journal of Nutritional Science and Vitaminology 2016;62(1):54‐61. - PubMed
Rubin 1996 {published data only}
-
- Rubin BK, Ramirez O, Ohar JA. Iodinated glycerol has no effect on pulmonary function, symptom score, or sputum properties in patients with stable chronic bronchitis. Chest 1996;109(2):348‐52. - PubMed
Saibene 2016 {published data only}
-
- Saibene F, Paone G, Lanata L, Puglisi G, Moscatelli B, Colli RD. Effect of carbocysteine lysine salt on frequency of exacerbations in COPD patient treated with or without inhaled steroids. European Respiratory Journal 2016;48:PA544. - PubMed
Salve 2016 {published data only}
Sushko 2015 {published data only}
-
- Sushko V, Shvaiko L, Bazyka K, Riazhska A. Treatment of chronic obstructive pulmonary disease in clean‐up workers of the Chornobyl NPP accident in the remote period after irradiation with additional 6 month prescription combination of ambroxol and essential phospholipids. European Respiratory Journal 2016;48:PA4299.
-
- Sushko VO, Shvaiko LI, Bazyka KD, Riazhska AS. Optimization of chronic obstructive pulmonary disease treatment in clean‐up workers of the Chornobyl NPP accident in the remote period after irradiation. Problemy Radiatsiinoi Medytsyny Ta Radiobiolohii 2015;20:457‐66. - PubMed
Tatsumi 2007a {published data only}
-
- Tatsumi K, Fukuchi Y. Carbocisteine improves quality of life in patients with chronic obstructive pulmonary disease. Journal of the American Geriatrics Society 2007;55(11):1884‐5. - PubMed
Tatsumi 2007b {published data only}
-
- Tatsumi K, Fukuchi Y. Carbocisteine reduces the frequency of exacerbations in patients with COPD: findings from the PEACE study. American Thoracic Society International Conference; 2007; May 18‐23; San Francisco. 2007:C97.
Velazquez 2001 {published data only}
-
- Velazquez A, Aguilar G, Sanchez C, Ochoa L, Sansores R, Remirez‐Venegas A. The mucolytic effect on quality of life in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A57.
Wilhelmi 2010 {published data only}
-
- Wilhelmi E. Treatment of COPD: benefit of 1.8 cineole as co medication confirmed [Behandlung der COPD: nutzen voan 1.8‐cineol als zusatztherapie bestatigt]. Journal Pharmakol U Ther 2010;2:554‐5.
References to studies awaiting assessment
CTRI/2015/01/005432 {published data only}
-
- Hussain A, Rizvi W. Comparative Evaluation of Efficacy and Safety Profile of Antioxidants‐N‐Acetyl‐L‐Cysteine(NAC) and Superoxide Dismutase (SOD) Supplementation in Patients of COPD. Clinical Trials Registry ‐ India 2015.
References to ongoing studies
ChiCTR1800016712 {published data only}
-
- Early Intervention With Carbocysteine and Low Dose Theophylline in Chinese Patients With Chronic Obstructive Pulmonary Disease. Chinese Clinical Trials Registry 2018. [ChiCTR1800016712]
ChiCTR‐IIR‐17012604 {published data only}
-
- Zhou Y, Ran P. Long‐Term Regular Treatment of Early COPD With Randomized, Double‐Blind, Placebo‐Controlled Multicenter Clinical Study With Acetylcysteine Effervescent Tablets. Chinese Clinical Trial Registry 2017. [ChiCTR‐IIR‐17012604 ]
Additional references
Anzueto 1997
-
- Anzueto A, Jubran A, Ohar JA, Piquette CA, Rennard SI, Colice G. Effects of aerosolized surfactant in patients with stable chronic bronchitis: a prospective randomized controlled trial. Journal of American Medical Association 1997;278(17):1426–31. - PubMed
Ballabio 2008
-
- Ballabio M, Nicola M, Moretti M. Long‐term use of antioxidant mucolytic erdosteine is especially beneficial in patient with more severe COPD. European Respiratory Society 18th Annual Congress; 2008 Oct 3‐7; Berlin. 2008:abstract ERS08L1_678.
Cates 2002
Cazzola 2015
Collet 1997
-
- Collet JP, Shapiro P, Ernst P, Renzi T, Ducruet T, Robinson A. Effects of an immunostimulating agent on acute exacerbations and hospitalizations in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1997;156(6):1719‐24. - PubMed
Criner 2015
Fowdar 2017
-
- Fowdar K, Chen H, He Z, Zhang J, Zhong X, Zhang J, et al. The effect of N‐acetylcysteine on exacerbations of chronic obstructive pulmonary disease: a meta‐analysis and systematic review. Heart and Lung 2017;46(2):120‐8. - PubMed
GOLD 2019
-
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2019. goldcopd.org/wp‐content/uploads/2018/11/GOLD‐2019‐v1.7‐FINAL‐14Nov2018‐W... (accessed before 14 December 2018).
Grandjean 2000
-
- Grandjean EM, Berthet PH, Ruffman R, Leuenberger PH. Efficacy of oral long‐term N‐acetylcysteine in chronic bronchopulmonary disease: a meta‐analysis of published double‐blind, placebo‐controlled clinical trials. Clinical Therapeutics 2000;22(2):209‐20. - PubMed
Grandjean 2000a
-
- Grandjean EM, Berthet PH, Ruffman R, Leuenberger PH. Cost‐effectiveness analysis of oral N‐acetylcysteine as a preventive treatment in chronic bronchitis. Pharmacology Research 2000;42(1):39‐50. - PubMed
Guyatt 1987
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. [Available from www.handbook.cochrane.org]
Jones 1992
-
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self‐complete measure of health status for chronic airflow limitation: the St. George's Respiratory Questionnaire. American Review of Respiratory Disease 1992;145(6):1321‐7. - PubMed
Jones 2005
-
- Jones PW. St. George's Respiratory Questionnaire: MCID. COPD 2005;2(1):75‐9. - PubMed
Jones 2013
-
- Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. American Journal of Respiratory and Critical Care Medicine 2013;189(3):250‐5. - PubMed
Li 2015
-
- Li X, Sun H, Liu C, Kang J. Mucolytic and antioxidant agents for exacerbations of chronic obstructive pulmonary disease: a meta‐analysis. Chinese Journal of Tuberculosis and Respiratory Diseases 2015;38(8):600‐6. - PubMed
Moretti 2007
-
- Moretti M, Nicola M. Prevention of COPD exacerbations and reduction of health care utilization with erdosteine. European Respiratory Journal 2007;30(Suppl 51):557s.
NICE 2018
-
- National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. www.nice.org.uk/guidance/ng115 (accessed before 14 December 2018).
Qaseem 2011
-
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Annals of Internal Medicine 2011;155(3):179‐91. - PubMed
Rahman 2005
-
- Rahman I. Oxidative stress in the pathogenesis of chronic obstructive pulmonary disease; cellular and molecular mechanisms. Cell Biochemistry and Biophysics 2005;43(1):167‐88. - PubMed
Rennard 2006
-
- Rennard SI, Vestbo J. COPD: the dangerous underestimate of 15%. Lancet 2006;367(9518):1216‐9. - PubMed
Rubin 2007
-
- Rubin BK. Mucolytics, expectorants, and mucokinetic medications. Respiratory Care 2007;52:859–65. - PubMed
Rubin 2014
Stey 2000
-
- Stey C, Steurer J, Bachmann S, Medici TC, Tramer MR. The effect of oral N‐acetylcysteine in chronic bronchitis: a quantitative systematic review. European Respiratory Journal 2000;16(2):253‐62. - PubMed
van Agteren 2016
Wedzicha 2017
-
- Wedzicha JA, Calverley PMA, Albert RK, Anzueto A, Criner GJ, Hurst JR, et al. Prevention of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. European Respiratory Journal 2017;50(3):pii: 1602265. - PubMed
WHO 2017
-
- World Health Organization. The top 10 causes of death. www.who.int/mediacentre/factsheets/fs310/en/ (accessed before 14 December 2018).
Yang 2018
-
- Yang IA, Brown JL, George J, Jenkins S, McDonald CF, McDonald V, et al. The COPD‐X Plan: Australian and New Zealand Guidelines for the Management of Chronic Obstructive Pulmonary Disease. Version 2.53. copdx.org.au/wp‐content/uploads/2018/11/COPDX‐V2‐55‐Aug‐2018.pdf (accessed before 13 December 2018).
References to other published versions of this review
Poole 1998
-
- Poole PJ, Black PN. The effect of mucolytic agents on exacerbation frequency in chronic bronchitis. Cochrane Database of Systematic Reviews 1998, Issue 4. [DOI: 10.1002/14651858.CD001287] - DOI
Poole 1999
-
- Poole PJ, Black PN. Mucolytic agents for chronic bronchitis. Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI: 10.1002/14651858.CD001287] - DOI
Poole 2001
Poole 2006
Poole 2012
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

