Pediatric intensive care unit admission due to respiratory syncytial virus: Retrospective multicenter study
- PMID: 31107995
- PMCID: PMC7168019
- DOI: 10.1111/ped.13893
Pediatric intensive care unit admission due to respiratory syncytial virus: Retrospective multicenter study
Abstract
Background: We investigated the characteristics and clinical outcomes of respiratory syncytial virus (RSV)-related pediatric intensive care unit (PICU) hospitalization and assessed the palivizumab (PZ) prophylaxis eligibility according to different guidelines from Korea, EU, and USA.
Methods: In this multicenter study, children <18 years of age hospitalized in six PICU from different hospitals due to severe RSV infection between September 2008 and March 2013 were included. A retrospective chart review was performed.
Results: A total of 92 patients were identified. The median length of PICU stay was 6 days (range, 1-154 days) and median PICU care cost was USD2,741 (range, USD556-98 243). Of 62 patients who were <2 years old at the beginning of the RSV season, 33 (53.2%) were high-risk patients for severe RSV infection. Hemodynamically significant congenital heart disease (22.6%) was the most common risk factor, followed by chronic lung disease (11.3%), neuromuscular disease or congenital abnormality of the airway (NMD/CAA) (11.3%), and prematurity (8.1%). The percentage of patients eligible for PZ prophylaxis ranged from 38.7% to 48.4% based on the guidelines, but only two (2.2%) received PZ ≤30 days prior to PICU admission. The median duration of mechanical ventilation was longer in children with NDM/CAA than in those without risk factors (26 days; range, 24-139 days vs 6 days, range, 2-68 days, P = 0.033). RSV-attributable mortality was 5.4%.
Conclusions: Children <2 years old with already well-known high risks represent a significant proportion of RSV-related PICU admissions. Increasing of the compliance for PZ prophylaxis practice among physicians is needed. Further studies are needed to investigate the burden of RSV infection in patients hospitalized in PICU, including children with NMD/CAA.
Keywords: eligibility; guideline; neuromuscular disorder or congenital abnormality of the airway; pediatric intensive care unit; respiratory syncytial virus.
© 2019 Japan Pediatric Society.
Figures
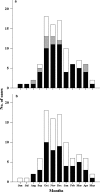
 , 12–24 months; □, >24 months); and (b) presence of risk factors (■, risk; □, no risk). (a) Sixty‐nine of 92 cases (75.0%) occurred in children <2 years of age at the time of
, 12–24 months; □, >24 months); and (b) presence of risk factors (■, risk; □, no risk). (a) Sixty‐nine of 92 cases (75.0%) occurred in children <2 years of age at the time of 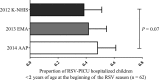
Similar articles
-
Palivizumab prophylaxis of respiratory syncytial virus disease from 1998 to 2002: results from four years of palivizumab usage.Pediatr Infect Dis J. 2003 Feb;22(2 Suppl):S46-54. doi: 10.1097/01.inf.0000053885.34703.84. Pediatr Infect Dis J. 2003. PMID: 12671452
-
Respiratory syncytial virus morbidity, premorbid factors, seasonality, and implications for prophylaxis.J Crit Care. 2012 Oct;27(5):464-8. doi: 10.1016/j.jcrc.2011.12.001. Epub 2012 Jan 9. J Crit Care. 2012. PMID: 22227087
-
Serum palivizumab level is associated with decreased severity of respiratory syncytial virus disease in high-risk infants.Hum Vaccin Immunother. 2014;10(10):2789-94. doi: 10.4161/hv.29635. Hum Vaccin Immunother. 2014. PMID: 25483663 Free PMC article. Clinical Trial.
-
Product review on the monoclonal antibody palivizumab for prevention of respiratory syncytial virus infection.Hum Vaccin Immunother. 2017 Sep 2;13(9):2138-2149. doi: 10.1080/21645515.2017.1337614. Epub 2017 Jun 12. Hum Vaccin Immunother. 2017. PMID: 28605249 Free PMC article. Review.
-
Disease burden of respiratory syncytial virus infection in the pediatric population in Japan.J Infect Chemother. 2022 Feb;28(2):146-157. doi: 10.1016/j.jiac.2021.11.007. Epub 2021 Dec 21. J Infect Chemother. 2022. PMID: 34952776 Review.
Cited by
-
Children born preterm admitted to paediatric intensive care for bronchiolitis: a systematic review and meta-analysis.BMC Pediatr. 2023 Jun 29;23(1):326. doi: 10.1186/s12887-023-04150-7. BMC Pediatr. 2023. PMID: 37386478 Free PMC article.
-
Hospital-based cross-sectional study on the clinical characteristics of children with severe acute respiratory infections in Hungary.BMC Infect Dis. 2024 Nov 9;24(1):1268. doi: 10.1186/s12879-024-10186-6. BMC Infect Dis. 2024. PMID: 39521980 Free PMC article.
-
Respiratory Syncytial Virus Infections in Pediatric Intensive Care: Association of Sociodemographic Data and Clinical Outcomes with Viral and Bacterial Co-infections.Turk Arch Pediatr. 2024 Sep 2;59(5):494-500. doi: 10.5152/TurkArchPediatr.2024.24149. Turk Arch Pediatr. 2024. PMID: 39440440 Free PMC article.
-
Respiratory Syncytial Virus Immunoprophylaxis with Palivizumab: 12-Year Observational Study of Usage and Outcomes in Canada.Am J Perinatol. 2022 Nov;39(15):1668-1677. doi: 10.1055/s-0041-1725146. Epub 2021 Mar 3. Am J Perinatol. 2022. PMID: 33657636 Free PMC article.
-
Trends in US Pediatric Hospital Admissions in 2020 Compared With the Decade Before the COVID-19 Pandemic.JAMA Netw Open. 2021 Feb 1;4(2):e2037227. doi: 10.1001/jamanetworkopen.2020.37227. JAMA Netw Open. 2021. PMID: 33576819 Free PMC article.
References
-
- Hall CB. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 2001; 344 (25): 1917–28. - PubMed
-
- Stockman LJ, Curns AT, Anderson LJ, Fischer‐Langley G. Respiratory syncytial virus‐associated hospitalizations among infants and young children in the United States, 1997‐2006. Pediatr. Infect. Dis. J. 2012; 31: 5–9. - PubMed
-
- Heikkinen T, Ojala E, Waris M. Clinical and socioeconomic burden of respiratory syncytial virus infection in children. J. Infect. Dis. 2017; 215: 17–23. - PubMed
-
- Butt ML, Symington A, Janes M, Elliott L, Steele S, Paes BA. The impact of prophylaxis on paediatric intensive care unit admissions for RSV infection: A retrospective, single‐centre study. Eur. J. Pediatr. 2011; 170: 907–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

