Environmental Cadmium Enhances Lung Injury by Respiratory Syncytial Virus Infection
- PMID: 31108100
- PMCID: PMC6717913
- DOI: 10.1016/j.ajpath.2019.04.013
Environmental Cadmium Enhances Lung Injury by Respiratory Syncytial Virus Infection
Abstract
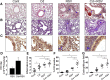
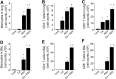
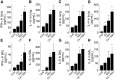
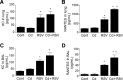
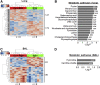
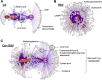
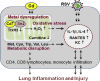
Cadmium (Cd) is a naturally occurring environmental toxicant that disrupts mitochondrial function at occupational exposure levels. The impacts of Cd exposure at low levels through dietary intake remain largely uncharacterized. Human respiratory syncytial virus (RSV) causes severe morbidity, which can require hospitalization and result in death in young children and elderly populations. The impacts of environmental Cd exposure on the severity of RSV disease are unknown. Herein, we used a mouse model to examine whether Cd pre-exposure at a level of dietary intake potentiates pulmonary inflammation on subsequent infection with RSV. Mice were given Cd or saline in drinking water for 28 days. Subsets of these mice were infected with RSV at 5 days before the end of the study. Cd pre-exposure caused relatively subtle changes in lung; however, it elevated the IL-4 level and altered metabolites associated with fatty acid metabolism. After RSV infection, mice pre-exposed to Cd had elevated lung RSV titer and increased inflammation, as measured by histopathology, immune cell infiltration, cytokines, and chemokines. RSV infection after Cd pre-exposure also caused widespread perturbation in metabolism of glycerophospholipids and amino acids (Trp, Met, and Cys, branched-chain amino acids), as well as carnitine shuttle associated with mitochondrial energy metabolism. The results show that Cd burden by dietary intake potentiates RSV infection and severe disease with associated mitochondrial metabolic disruption.
Copyright © 2019 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Low-Dose Cadmium Potentiates Metabolic Reprogramming Following Early-Life Respiratory Syncytial Virus Infection.Toxicol Sci. 2022 Jun 28;188(1):62-74. doi: 10.1093/toxsci/kfac049. Toxicol Sci. 2022. PMID: 35512398 Free PMC article.
-
Suppression of IRG-1 Reduces Inflammatory Cell Infiltration and Lung Injury in Respiratory Syncytial Virus Infection by Reducing Production of Reactive Oxygen Species.J Virol. 2016 Jul 27;90(16):7313-7322. doi: 10.1128/JVI.00563-16. Print 2016 Aug 15. J Virol. 2016. PMID: 27252532 Free PMC article.
-
Ultrafine carbon black particles enhance respiratory syncytial virus-induced airway reactivity, pulmonary inflammation, and chemokine expression.Toxicol Sci. 2003 Apr;72(2):339-46. doi: 10.1093/toxsci/kfg032. Epub 2003 Mar 7. Toxicol Sci. 2003. PMID: 12655033
-
Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy--a review.Virus Genes. 2006 Oct;33(2):235-52. doi: 10.1007/s11262-006-0064-x. Virus Genes. 2006. PMID: 16972040 Review.
-
RSV Infection and Neurodegenerative Diseases: A Hypothesis of Energy Metabolism Disruption via the Lung-Brain Axis.Aging Dis. 2025 Jun 2. doi: 10.14336/AD.2025.0534. Online ahead of print. Aging Dis. 2025. PMID: 40479567 Review.
Cited by
-
Environmental Cadmium and Mortality from Influenza and Pneumonia in U.S. Adults.Environ Health Perspect. 2020 Dec;128(12):127004. doi: 10.1289/EHP7598. Epub 2020 Dec 16. Environ Health Perspect. 2020. PMID: 33325772 Free PMC article.
-
Redox organization of living systems.Free Radic Biol Med. 2024 May 1;217:179-189. doi: 10.1016/j.freeradbiomed.2024.03.008. Epub 2024 Mar 14. Free Radic Biol Med. 2024. PMID: 38490457 Free PMC article. Review.
-
Low-Dose Cadmium Potentiates Metabolic Reprogramming Following Early-Life Respiratory Syncytial Virus Infection.Toxicol Sci. 2022 Jun 28;188(1):62-74. doi: 10.1093/toxsci/kfac049. Toxicol Sci. 2022. PMID: 35512398 Free PMC article.
-
Titanium dioxide nanoparticles exaggerate respiratory syncytial virus-induced airway epithelial barrier dysfunction.Am J Physiol Lung Cell Mol Physiol. 2020 Sep 1;319(3):L481-L496. doi: 10.1152/ajplung.00104.2020. Epub 2020 Jul 8. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32640839 Free PMC article.
-
Omics Integration for Mitochondria Systems Biology.Antioxid Redox Signal. 2020 Apr 20;32(12):853-872. doi: 10.1089/ars.2019.8006. Epub 2020 Feb 3. Antioxid Redox Signal. 2020. PMID: 31891667 Free PMC article. Review.
References
-
- Falsey A.R., Hennessey P.A., Formica M.A., Cox C., Walsh E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. - PubMed
-
- Hall C.B., Weinberg G.A., Blumkin A.K., Edwards K.M., Staat M.A., Schultz A.F., Poehling K.A., Szilagyi P.G., Griffin M.R., Williams J.V., Zhu Y., Grijalva C.G., Prill M.M., Iwane M.K. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132:e341–e348. - PubMed
-
- Nair H., Nokes D.J., Gessner B.D., Dherani M., Madhi S.A., Singleton R.J., O'Brien K.L., Roca A., Wright P.F., Bruce N., Chandran A., Theodoratou E., Sutanto A., Sedyaningsih E.R., Ngama M., Munywoki P.K., Kartasasmita C., Simoes E.A., Rudan I., Weber M.W., Campbell H. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. - PMC - PubMed
-
- Knudson C.J., Varga S.M. The relationship between respiratory syncytial virus and asthma. Vet Pathol. 2015;52:97–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

