Cholesterol enrichment in liver mitochondria impairs oxidative phosphorylation and disrupts the assembly of respiratory supercomplexes
- PMID: 31108462
- PMCID: PMC6526464
- DOI: 10.1016/j.redox.2019.101214
Cholesterol enrichment in liver mitochondria impairs oxidative phosphorylation and disrupts the assembly of respiratory supercomplexes
Abstract
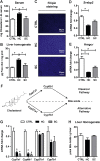
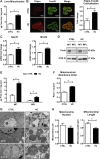
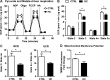
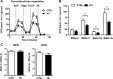
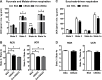
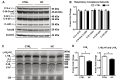
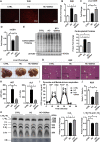
Mitochondrial cholesterol accumulation is a hallmark of alcoholic and non-alcoholic fatty liver diseases and impairs the function of specific solute carriers through changes in membrane physical properties. However, its impact on mitochondrial respiration and organization of respiratory supercomplexes has not been determined so far. Here we fed mice a cholesterol-enriched diet (HC) supplemented with sodium cholate to examine the effect of cholesterol in mitochondrial function. HC feeding increased liver cholesterol content, which downregulated Srebp2 and Hmgcr expression, while sodium cholate administration decreased Cyp7a1 and Cyp8b1 mRNA levels, suggesting the downregulation of bile acid synthesis through the classical pathway. HC-fed mice exhibited increased expression of Stard1 and Mln64 and enhanced mitochondrial free cholesterol levels (2-3 fold), leading to decreased membrane fluidity. Mitochondria from HC-fed mice displayed increased cholesterol loading in both outer and inner mitochondrial membranes. Cholesterol loading decreased complex I and complex II-driven state 3 respiration and mitochondrial membrane potential. Decreased respiratory and uncoupling control ratio from complex I was also observed after in situ enrichment of mouse liver mitochondria with cholesterol or enantiomer cholesterol, the mirror image of natural cholesterol. Moreover, in vivo cholesterol loading decreased the level of complex III2 and the assembly of respiratory supercomplexes I1+III2+IV and I1+III2. Moreover, HC feeding caused oxidative stress and mitochondrial GSH (mGSH) depletion, which translated in hepatic steatosis and liver injury, effects that were rescued by replenishing mGSH with GSH ethyl ester. Overall, mitochondrial cholesterol accumulation disrupts mitochondrial functional performance and the organization of respiratory supercomplexes assembly, which can contribute to oxidative stress and liver injury.
Keywords: Cholesterol; Hepatic diseases; Liver; Mitochondria; Oxidative stress; Respiration.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
The 2-oxoglutarate carrier promotes liver cancer by sustaining mitochondrial GSH despite cholesterol loading.Redox Biol. 2018 Apr;14:164-177. doi: 10.1016/j.redox.2017.08.022. Epub 2017 Sep 14. Redox Biol. 2018. PMID: 28942194 Free PMC article.
-
High resolution respirometry analysis of polyethylenimine-mediated mitochondrial energy crisis and cellular stress: Mitochondrial proton leak and inhibition of the electron transport system.Biochim Biophys Acta. 2013 Oct;1827(10):1213-25. doi: 10.1016/j.bbabio.2013.07.001. Epub 2013 Jul 11. Biochim Biophys Acta. 2013. PMID: 23850549
-
Targeting cholesterol at different levels in the mevalonate pathway protects fatty liver against ischemia-reperfusion injury.J Hepatol. 2011 May;54(5):1002-10. doi: 10.1016/j.jhep.2010.08.031. Epub 2010 Oct 29. J Hepatol. 2011. PMID: 21145825
-
The function of the respiratory supercomplexes: the plasticity model.Biochim Biophys Acta. 2014 Apr;1837(4):444-50. doi: 10.1016/j.bbabio.2013.12.009. Epub 2013 Dec 22. Biochim Biophys Acta. 2014. PMID: 24368156 Review.
-
Respiratory chain supercomplexes in plant mitochondria.Plant Physiol Biochem. 2004 Dec;42(12):937-42. doi: 10.1016/j.plaphy.2004.09.010. Epub 2005 Jan 21. Plant Physiol Biochem. 2004. PMID: 15707832 Review.
Cited by
-
Diabetes Mellitus, Mitochondrial Dysfunction and Ca2+-Dependent Permeability Transition Pore.Int J Mol Sci. 2020 Sep 8;21(18):6559. doi: 10.3390/ijms21186559. Int J Mol Sci. 2020. PMID: 32911736 Free PMC article. Review.
-
Organic and Inorganic Selenium Compounds Affected Lipidomic Profile of Spleen of Lambs Fed with Diets Enriched in Carnosic Acid and Fish Oil.Animals (Basel). 2023 Dec 29;14(1):133. doi: 10.3390/ani14010133. Animals (Basel). 2023. PMID: 38200864 Free PMC article.
-
Expression of Synj2bp in mouse liver regulates the extent of wrappER-mitochondria contact to maintain hepatic lipid homeostasis.Biol Direct. 2022 Dec 1;17(1):37. doi: 10.1186/s13062-022-00344-8. Biol Direct. 2022. PMID: 36457006 Free PMC article.
-
Dysregulation of mannose-6-phosphate-dependent cholesterol homeostasis in acinar cells mediates pancreatitis.J Clin Invest. 2021 Aug 2;131(15):e146870. doi: 10.1172/JCI146870. J Clin Invest. 2021. PMID: 34128834 Free PMC article.
-
Warburg's Ghost-Cancer's Self-Sustaining Phenotype: The Aberrant Carbon Flux in Cholesterol-Enriched Tumor Mitochondria via Deregulated Cholesterogenesis.Front Cell Dev Biol. 2021 Mar 12;9:626316. doi: 10.3389/fcell.2021.626316. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33777935 Free PMC article. Review.
References
-
- Horton J.D., Goldstein J.L., Brown M.S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002;109:1125–1131. - PMC - PubMed
- Horton JD., Goldstein JL., Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 2002; 109: 1125-1131. - PMC - PubMed

