Hypoxia-Ischemia and Hypothermia Independently and Interactively Affect Neuronal Pathology in Neonatal Piglets with Short-Term Recovery
- PMID: 31108487
- PMCID: PMC6732227
- DOI: 10.1159/000496602
Hypoxia-Ischemia and Hypothermia Independently and Interactively Affect Neuronal Pathology in Neonatal Piglets with Short-Term Recovery
Abstract
Therapeutic hypothermia is the standard of clinical care for moderate neonatal hypoxic-ischemic encephalopathy. We investigated the independent and interactive effects of hypoxia-ischemia (HI) and temperature on neuronal survival and injury in basal ganglia and cerebral cortex in neonatal piglets. Male piglets were randomized to receive HI injury or sham procedure followed by 29 h of normothermia, sustained hypothermia induced at 2 h, or hypothermia with rewarming during fentanyl-nitrous oxide anesthesia. Viable and injured neurons and apoptotic profiles were counted in the anterior putamen, posterior putamen, and motor cortex at 29 h after HI injury or sham procedure. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) identified genomic DNA fragmentation to confirm cell death. Though hypothermia after HI preserved viable neurons in the anterior and posterior putamen, hypothermia prevented neuronal injury in only the anterior putamen. Hypothermia initiated 2 h after injury did not protect against apoptotic cell death in either the putamen or motor cortex, and rewarming from hypothermia was associated with increased apoptosis in the motor cortex. In non-HI shams, sustained hypothermia during anesthesia was associated with neuronal injury and corresponding viable neuron loss in the anterior putamen and motor cortex. TUNEL confirmed increased neurodegeneration in the putamen of hypothermic shams. Anesthetized, normothermic shams did not show abnormal neuronal cytopathology in the putamen or motor cortex, thereby demonstrating minimal contribution of the anesthetic regimen to neuronal injury during normothermia. We conclude that the efficacy of hypothermic protection after HI is region specific and that hypothermia during anesthesia in the absence of HI may be associated with neuronal injury in the developing brain. Studies examining the potential interactions between hypothermia and anesthesia, as well as with longer durations of hypothermia, are needed.
Keywords: Brain injury; Hypothermia therapy; Hypoxic-ischemic encephalopathy; Neonatal; Neurodegeneration; Neuroprotection; Perinatal asphyxia.
© 2019 S. Karger AG, Basel.
Conflict of interest statement
Disclosure Statement
The authors declare no conflicts of interest.
Figures



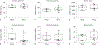

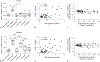
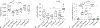
Similar articles
-
White matter apoptosis is increased by delayed hypothermia and rewarming in a neonatal piglet model of hypoxic ischemic encephalopathy.Neuroscience. 2016 Mar 1;316:296-310. doi: 10.1016/j.neuroscience.2015.12.046. Epub 2015 Dec 29. Neuroscience. 2016. PMID: 26739327 Free PMC article.
-
Hypothermia and Rewarming Activate a Macroglial Unfolded Protein Response Independent of Hypoxic-Ischemic Brain Injury in Neonatal Piglets.Dev Neurosci. 2016;38(4):277-294. doi: 10.1159/000448585. Epub 2016 Sep 14. Dev Neurosci. 2016. PMID: 27622292 Free PMC article.
-
Rewarming from therapeutic hypothermia induces cortical neuron apoptosis in a swine model of neonatal hypoxic-ischemic encephalopathy.J Cereb Blood Flow Metab. 2015 May;35(5):781-93. doi: 10.1038/jcbfm.2014.245. Epub 2015 Jan 7. J Cereb Blood Flow Metab. 2015. PMID: 25564240 Free PMC article.
-
Mechanisms of hypothermic neuroprotection.Clin Perinatol. 2014 Mar;41(1):161-75. doi: 10.1016/j.clp.2013.10.005. Epub 2013 Dec 12. Clin Perinatol. 2014. PMID: 24524453 Review.
-
Advances in the Prevention and Treatment of Neonatal Hypothermia in Early Birth.Ther Hypothermia Temp Manag. 2022 Jun;12(2):51-56. doi: 10.1089/ther.2021.0036. Epub 2022 Apr 5. Ther Hypothermia Temp Manag. 2022. PMID: 35384724 Review.
Cited by
-
Disrupted brain connectivity in children treated with therapeutic hypothermia for neonatal encephalopathy.Neuroimage Clin. 2021;30:102582. doi: 10.1016/j.nicl.2021.102582. Epub 2021 Feb 10. Neuroimage Clin. 2021. PMID: 33636541 Free PMC article.
-
Motor function and white matter connectivity in children cooled for neonatal encephalopathy.Neuroimage Clin. 2021;32:102872. doi: 10.1016/j.nicl.2021.102872. Epub 2021 Nov 3. Neuroimage Clin. 2021. PMID: 34749285 Free PMC article.
-
Umbilical cord blood troponin I, myoglobin and CK-MB in neonatal hypoxic ischemic encephalopathy and the clinical significance.Exp Ther Med. 2020 Jan;19(1):545-550. doi: 10.3892/etm.2019.8248. Epub 2019 Nov 26. Exp Ther Med. 2020. PMID: 31885699 Free PMC article.
-
Spatial T-maze identifies cognitive deficits in piglets 1 month after hypoxia-ischemia in a model of hippocampal pyramidal neuron loss and interneuron attrition.Behav Brain Res. 2019 Sep 2;369:111921. doi: 10.1016/j.bbr.2019.111921. Epub 2019 Apr 19. Behav Brain Res. 2019. PMID: 31009645 Free PMC article.
-
Hypothermic Protection in Neocortex Is Topographic and Laminar, Seizure Unmitigating, and Partially Rescues Neurons Depleted of RNA Splicing Protein Rbfox3/NeuN in Neonatal Hypoxic-Ischemic Male Piglets.Cells. 2023 Oct 15;12(20):2454. doi: 10.3390/cells12202454. Cells. 2023. PMID: 37887298 Free PMC article.
References
-
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al.; National Institute of Child Health and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005. October;353(15):1574–84. - PubMed
-
- Lodygensky GA, Battin MR, Gunn AJ. Mild neonatal encephalopathy-how, when, and how much to treat? JAMA Pediatr. 2018. January;172(1):3–4. - PubMed
-
- Oliveira V, Singhvi DP, Montaldo P, Lally PJ, Mendoza J, Manerkar S, et al. Therapeutic hypothermia in mild neonatal encephalopathy: a national survey of practice in the UK. Arch Dis Child Fetal Neonatal Ed. 2018. July;103(4):F388–90. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

