Site-specific abnormalities in the visual system of a mouse model of CDKL5 deficiency disorder
- PMID: 31108505
- PMCID: PMC6736061
- DOI: 10.1093/hmg/ddz102
Site-specific abnormalities in the visual system of a mouse model of CDKL5 deficiency disorder
Abstract
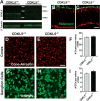
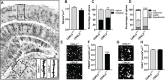
CDKL5 deficiency disorder (CDD) is a neurodevelopmental disorder characterized by a severe global developmental delay and early-onset seizures. Notably, patients show distinctive visual abnormalities often clinically diagnosed as cortical visual impairment. However, the involvement of cerebral cortical dysfunctions in the origin of the symptoms is poorly understood. CDD mouse models also display visual deficits, and cortical visual responses can be used as a robust biomarker in CDKL5 mutant mice. A deeper understanding of the circuits underlying the described visual deficits is essential for directing preclinical research and translational approaches. Here, we addressed this question in two ways: first, we performed an in-depth morphological analysis of the visual pathway, from the retina to the primary visual cortex (V1), of CDKL5 null mice. We found that the lack of CDKL5 produced no alteration in the organization of retinal circuits. Conversely, CDKL5 mutants showed reduced density and altered morphology of spines and decreased excitatory synapse marker PSD95 in the dorsal lateral geniculate nucleus and in V1. An increase in the inhibitory marker VGAT was selectively present in V1. Second, using a conditional CDKL5 knockout model, we showed that selective cortical deletion of CDKL5 from excitatory cells is sufficient to produce abnormalities of visual cortical responses, demonstrating that the normal function of cortical circuits is dependent on CDKL5. Intriguingly, these deficits were associated with morphological alterations of V1 excitatory and inhibitory synaptic contacts. In summary, this work proposes cortical circuit structure and function as a critically important target for studying CDD.
© The Author(s) 2019. Published by Oxford University Press.
Figures




Similar articles
-
A GABAB receptor antagonist rescues functional and structural impairments in the perirhinal cortex of a mouse model of CDKL5 deficiency disorder.Neurobiol Dis. 2021 Jun;153:105304. doi: 10.1016/j.nbd.2021.105304. Epub 2021 Feb 20. Neurobiol Dis. 2021. PMID: 33621640
-
Adult-Onset Deletion of CDKL5 in Forebrain Glutamatergic Neurons Impairs Synaptic Integrity and Behavior in Mice.Int J Mol Sci. 2025 Jul 10;26(14):6626. doi: 10.3390/ijms26146626. Int J Mol Sci. 2025. PMID: 40724873 Free PMC article.
-
Aged heterozygous Cdkl5 mutant mice exhibit spontaneous epileptic spasms.Exp Neurol. 2020 Oct;332:113388. doi: 10.1016/j.expneurol.2020.113388. Epub 2020 Jun 22. Exp Neurol. 2020. PMID: 32585155 Free PMC article.
-
Molecular and Synaptic Bases of CDKL5 Disorder.Dev Neurobiol. 2019 Jan;79(1):8-19. doi: 10.1002/dneu.22639. Epub 2018 Oct 19. Dev Neurobiol. 2019. PMID: 30246934 Review.
-
Cyclin-Dependent Kinase-Like 5 (CDKL5): Possible Cellular Signalling Targets and Involvement in CDKL5 Deficiency Disorder.Neural Plast. 2020 Jun 5;2020:6970190. doi: 10.1155/2020/6970190. eCollection 2020. Neural Plast. 2020. PMID: 32587608 Free PMC article. Review.
Cited by
-
Temporal manipulation of Cdkl5 reveals essential postdevelopmental functions and reversible CDKL5 deficiency disorder-related deficits.J Clin Invest. 2021 Oct 15;131(20):e143655. doi: 10.1172/JCI143655. J Clin Invest. 2021. PMID: 34651584 Free PMC article.
-
Inhibition of microglia overactivation restores neuronal survival in a mouse model of CDKL5 deficiency disorder.J Neuroinflammation. 2021 Jul 8;18(1):155. doi: 10.1186/s12974-021-02204-0. J Neuroinflammation. 2021. PMID: 34238328 Free PMC article.
-
Cyclocreatine treatment ameliorates the cognitive, autistic and epileptic phenotype in a mouse model of Creatine Transporter Deficiency.Sci Rep. 2020 Oct 27;10(1):18361. doi: 10.1038/s41598-020-75436-4. Sci Rep. 2020. PMID: 33110151 Free PMC article.
-
A Novel Mouse Model Unveils Protein Deficiency in Truncated CDKL5 Mutations.Neurosci Bull. 2025 May;41(5):805-820. doi: 10.1007/s12264-024-01346-4. Epub 2025 Mar 5. Neurosci Bull. 2025. PMID: 40042769 Free PMC article.
-
Independent genetic strategies define the scope and limits of CDKL5 deficiency disorder reversal.Cell Rep Med. 2025 Feb 18;6(2):101926. doi: 10.1016/j.xcrm.2024.101926. Epub 2025 Jan 23. Cell Rep Med. 2025. PMID: 39855191 Free PMC article.
References
-
- Barber A.J. and Baccouche B. (2017) Neurodegeneration in diabetic retinopathy: potential for novel therapies. Vision Res., 139(October), 82–92. - PubMed
-
- Boggio E.M., Pancrazi L., Gennaro M., Lo Rizzo C., Mari F., Meloni I., Ariani F., Panighini A., Novelli E., Biagioni M. et al. (2016) Visual impairment in FOXG1-mutated individuals and mice. Neuroscience, 324(June), 496–508. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

