The Effect of Sevelamer on Serum Levels of Gut-Derived Uremic Toxins: Results from In Vitro Experiments and A Multicenter, Double-Blind, Placebo-Controlled, Randomized Clinical Trial
- PMID: 31109001
- PMCID: PMC6563242
- DOI: 10.3390/toxins11050279
The Effect of Sevelamer on Serum Levels of Gut-Derived Uremic Toxins: Results from In Vitro Experiments and A Multicenter, Double-Blind, Placebo-Controlled, Randomized Clinical Trial
Abstract
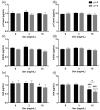
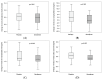
High serum levels of gut-derived uremic toxins, especially p-cresyl sulfate (pCS), indoxyl sulfate (IS) and indole acetic acid (IAA), have been linked to adverse outcomes in patients with chronic kidney disease (CKD). Sevelamer carbonate could represent an interesting option to limit the elevation of gut-derived uremic toxins. The aim of the present study was to evaluate the adsorptive effect of sevelamer carbonate on different gut-derived protein-bound uremic toxins or their precursors in vitro, and its impact on the serum levels of pCS, IS and IAA in patients with CKD stage 3b/4. For the in vitro experiments, IAA, p-cresol (precursor of pCS) and indole (precursor of IS), each at a final concentration of 1 or 10 µg/mL, were incubated in centrifugal 30 kDa filter devices with 3 or 15 mg/mL sevelamer carbonate in phosphate-buffered saline at a pH adjusted to 6 or 8. Then, samples were centrifuged and free uremic toxins in the filtrates were analyzed. As a control experiment, the adsorption of phosphate was also evaluated. Additionally, patients with stage 3b/4 CKD (defined as an eGFR between 15 and 45 mL/min per 1.73 m2) were included in a multicenter, double-blind, placebo-controlled, randomized clinical trial. The participants received either placebo or sevelamer carbonate (4.8 g) three times a day for 12 weeks. The concentrations of the toxins and their precursors were measured using a validated high-performance liquid chromatography method with a diode array detector. In vitro, regardless of the pH and concentration tested, sevelamer carbonate did not show adsorption of indole and p-cresol. Conversely, with 10 µg/mL IAA, use of a high concentration of sevelamer carbonate (15 mg/mL) resulted in a significant toxin adsorption both at pH 8 (mean reduction: 26.3 ± 3.4%) and pH 6 (mean reduction: 38.7 ± 1.7%). In patients with CKD stage 3b/4, a 12-week course of treatment with sevelamer carbonate was not associated with significant decreases in serum pCS, IS and IAA levels (median difference to baseline levels: -0.12, 0.26 and -0.06 µg/mL in the sevelamer group vs. 1.97, 0.38 and 0.05 µg/mL in the placebo group, respectively). Finally, in vitro, sevelamer carbonate was capable of chelating a gut-derived uremic toxin IAA but not p-cresol and indole, the precursors of pCS and IS in the gut. In a well-designed clinical study of patients with stage 3b/4 CKD, a 12-week course of treatment with sevelamer carbonate was not associated with significant changes in the serum concentrations of pCS, IS and IAA.
Keywords: sevelamer; uremic toxins.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Vanholder R., De Smet R., Glorieux G., Argiles A., Baurmeister U., Brunet P., Clark W., Cohen G., De Deyn P.P., Deppisch R., et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–1943. doi: 10.1046/j.1523-1755.2003.00924.x. - DOI - PubMed
-
- Morimoto K., Tominaga Y., Agatsuma Y., Miyamoto M., Kashiwagura S., Takahashi A., Sano Y., Yano K., Kakinuma C., Ogihara T., et al. Intestinal secretion of indoxyl sulfate as a possible compensatory excretion pathway in chronic kidney disease. Biopharm. Drug Dispos. 2018;39:328–334. doi: 10.1002/bdd.2149. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

